Lessons from "real life experience" of rifaximin use in the management of recurrent hepatic encephalopathy
- PMID: 31984117
- PMCID: PMC6946626
- DOI: 10.4254/wjh.v12.i1.10
Lessons from "real life experience" of rifaximin use in the management of recurrent hepatic encephalopathy
Abstract
Background: Hepatic encephalopathy (HE) is a major complication of cirrhosis with independent prognostic significance. The current management of HE is mainly based on lactulose. Rifaximin has been shown to decrease the risk of HE recurrence in patients with episodic forms. HE can also be persistent. However, there is no drug support recommendation for rifaximin use in this setting.
Aim: To assess the effectiveness of rifaximin in the management of recurrent episodes of HE and recurrent acute exacerbations on persistent HE, in "real life conditions".
Methods: In this retrospective study, using a within-subjects design, we collected data of patients treated with rifaximin for HE in two liver diseases centers, during the six-month period before and during the six-month period after the initiation of rifaximin. The primary effectiveness endpoint was the total number of HE events involving hospitalization.
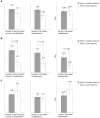
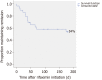
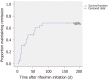
Results: Rifaximin was introduced for prevention of recurrent HE episodes in 29 out of 62 patients with normal mental status between episodes and for prevention of recurrent acute exacerbations on persistent HE in 33 out of 62 patients. In the "prevention of recurrent HE episodes" group, fewer HE events (0.79 vs 1.78; P = 0.013) were reported during the period of time when rifaximin was used. In the "prevention of recurrent acute exacerbations on persistent HE" group, there was no significant difference in the number of HE-events (1.48 vs 1.77; P = 0.582).
Conclusion: In this real-life experience, the effectiveness of rifaximin was confirmed in the prevention of HE episodes recurrence but was not proved in the prevention of acute exacerbations recurrence on persistent HE.
Keywords: Cirrhosis; Hepatic encephalopathy; Hospitalization; Liver disease; Rifaximin.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Bureau C and Cadranel JF have conflicts of interest with Alpha Wassermann as speakers at a symposium and Norgine as consultants. The remaining authors disclose no conflict.
Figures
References
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. - PubMed
-
- Zatoński WA, Sulkowska U, Mańczuk M, Rehm J, Boffetta P, Lowenfels AB, La Vecchia C. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res. 2010;16:193–201. - PubMed
-
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690–696. - PubMed
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. - PubMed
-
- Moscucci F, Nardelli S, Pentassuglio I, Pasquale C, Ridola L, Merli M, Riggio O. Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health-related quality of life in cirrhotic patients. Liver Int. 2011;31:1505–1510. - PubMed
LinkOut - more resources
Full Text Sources

