Theoretical Study of the Kinetics of the Gas-Phase Reaction between Phenyl and Amino Radicals
- PMID: 31984286
- PMCID: PMC6977206
- DOI: 10.1021/acsomega.9b03967
Theoretical Study of the Kinetics of the Gas-Phase Reaction between Phenyl and Amino Radicals
Abstract
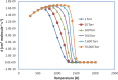
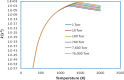
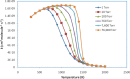
The potential energy surface (PES) of the C6H5 + NH2 reaction has been investigated by using ab initio CCSD(T)//B3LYP/6-311++G(3df,2p) calculations. The conventional transition-state theory (TST) and the variable reaction coordinate-TST (VRC-TST) have been used to predict the rate constants for the channels possessing tight and barrierless transition states, respectively. The Rice-Ramsperger-Kassel-Marcus/Master equation (RRKM/ME) theory has been utilized to determine the pressure-dependent rate constants for these reactions. The PES shows that the reaction begins with an exothermic barrierless addition of NH2 to C6H5 producing the vital intermediate state, namely, aniline (C6H5NH2, IS1). Once IS1 is generated, it can further isomerize to various intermediate states, which can give rise to different products, including PR4 (4,5,6-trihydro-1-amino phenyl + H2), PR5 (3,4,5,6-tetrahydro phenyl + NH3), PR6 (2,3,5,6-tetrahydro-1-imidogen phenyl + H2), PR9 (3,4,5,6-tetrahydro-1-imidogen phenyl + H2), and PR10 (2,5,6-trihydro-1-amino phenyl + H2), of which the most stable product, PR5, was formed by the most favorable channel going through the two advantageous transition states T1/11 (-28.9 kcal/mol) and T11P5 (-21.5 kcal/mol). The calculated rate constants for the low-energy channel, 1.37 × 10-9 and 2.16 × 10-11 cm3 molecule-1 s-1 at T = 300, P = 1 Torr and T = 2000 K, P = 760 Torr, respectively, show that the title reaction is almost pressure- and temperature-dependent. The negative temperature-dependent rate coefficients can be expressed in the modified Arrhenius form of k 1 = 8.54 × 1013 T -7.20 exp (-7.07 kcal·mol-1/RT) and k 2 = 2.42 × 1015 T -7.61 exp (-7.75 kcal·mol-1/RT) at 1 and 10 Torr, respectively, and in the temperature range of 300-2000 K. The forward and reverse rate coefficients as well as the high-pressure equilibrium constants of the C6H5 + NH2 ↔ IS1 process were also predicted; their values revealed that its kinetics do not depend on pressure at low temperature but strongly depend on pressure at high temperature. Moreover, the predicted formation enthalpies of reactants and the enthalpy changes of some channels are in good agreement with the experimental results.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Cherchneff I.; Barker J. R. Polycyclic aromatic hydrocarbons and molecular equilibria in carbon-rich stars. Astrophys. J. 1992, 394, 703–716. 10.1086/171624. - DOI
-
- Messenger S.; Amari S.; Gao X.; Walker R. M.; Clemett S. J.; Chillier X. D. F.; Zare R. N.; Lewis R. S. Indigenous polycyclic aromatic hydrocarbons in circumstellar graphite grains from primitive meteorites. Astrophys. J. 1998, 502, 284–295. 10.1086/305874. - DOI
-
- Allain T.; Sedlmayr E.; Leach S. PAHs in circumstellar envelopes. I. Processes affecting PAH formation and growth. Astron. Astrophys. 1997, 323, 163–176.
-
- Frenklach M.; Clary D. W.; Gardiner W. C.; Stein S. E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. Symp. (Int.) Combust. 1985, 20, 887–901. 10.1016/S0082-0784(85)80578-6. - DOI
-
- Kazakov A.; Frenklach M. On the relative contribution of acetylene and aromatics to soot particle surface growth. Combust. Flame 1998, 112, 270–274. 10.1016/S0010-2180(97)81776-2. - DOI
LinkOut - more resources
Full Text Sources

