Effects of Rivaroxaban on Biomarkers of Coagulation and Inflammation: A Post Hoc Analysis of the X-VeRT Trial
- PMID: 31984306
- PMCID: PMC6978177
- DOI: 10.1055/s-0040-1701206
Effects of Rivaroxaban on Biomarkers of Coagulation and Inflammation: A Post Hoc Analysis of the X-VeRT Trial
Abstract
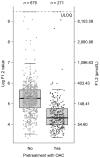
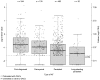
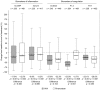
Introduction This X-VeRT (eXplore the efficacy and safety of once-daily oral riVaroxaban for the prevention of caRdiovascular events in patients with nonvalvular aTrial fibrillation scheduled for cardioversion) substudy evaluated the effects of treatment with rivaroxaban or a vitamin-K antagonist (VKA) on levels of biomarkers of coagulation (D-dimer, thrombin-antithrombin III complex [TAT] and prothrombin fragment [F1.2]) and inflammation (high sensitivity C-reactive protein [hs-CRP] and high-sensitivity interleukin-6 [hs-IL-6]) in patients with atrial fibrillation (AF) who were scheduled for cardioversion and had not received adequate anticoagulation at baseline (defined as, in the 21 days before randomization: no oral anticoagulant; international normalized ratio <2.0 with VKA treatment; or <80% compliance with non-VKA oral anticoagulant treatment). Methods Samples for biomarker analysis were taken at baseline ( n = 958) and treatment completion (42 days after cardioversion; n = 918). The influence of clinical characteristics on baseline biomarker levels and the effect of treatment on changes in biomarker levels were evaluated using linear and logistic models. Results Baseline levels of some biomarkers were significantly associated with type of AF (D-dimer and hs-IL-6) and with history of congestive heart failure (hs-CRP, D-dimer, and hs-IL-6). Rivaroxaban and VKA treatments were associated with reductions from baseline in levels of D-dimer (-32.3 and -37.6%, respectively), TAT (-28.0 and -23.1%, respectively), hs-CRP (-12.5 and -17.9%, respectively), and hs-IL-6 (-9.2 and -9.8%, respectively). F1.2 levels were reduced from baseline in patients receiving a VKA (-53.0%) but not in those receiving rivaroxaban (2.7%). Conclusion Anticoagulation with rivaroxaban reduced levels of key inflammation and coagulation biomarkers to a similar extent as VKAs, with the exception of F1.2. Further investigation to confirm the value of these biomarkers in patients with AF is merited.
Keywords: anticoagulants; atrial fibrillation; biomarkers; inflammation; rivaroxaban.
Conflict of interest statement
Conflict of Interest P.K. has received research support from the British Heart Foundation, the European Union, the German Centre for Cardiovascular Research, the Leducq Foundation and the Medical Research Council, and from several drug and device companies active in atrial fibrillation; he has also received honoraria from several such companies, including Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and Daiichi Sankyo. He is listed as an inventor on two patents held by the University of Birmingham (Atrial Fibrillation Therapy WO 2015140571; Markers for Atrial Fibrillation WO 2016012783). M.D.E. is a consultant and speaker for Boehringer Ingelheim and a consultant for Aegerion, Bayer, Bristol-Myers Squibb, Coherex, Daiichi Sankyo, Gilead, Janssen, Johnson & Johnson, Medtronic, Merck, Pfizer, Portola, Pozen, and Sanofi-Aventis. Y.P. has no conflict of interest to declare. A.J.C. has received personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, GlaxoSmithKline, Meda, Sanofi-Aventis, and Servier. S.H.H. has received consultancy and lecture fees from Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb/Pfizer, Cardiome, Gilead, Johnson & Johnson, Medtronic, Sanofi-Aventis, Servier and St. Jude Medical. R.C. has received consultancy fees from Abbott, Bayer, Biosense Webster, Boehringer Ingelheim, Boston Scientific, ELA Sorin, Medtronic, Pfizer, and St. Jude Medical; speaker's bureau fees from Abbott, Bard, Bayer, Biosense Webster, Boehringer Ingelheim, Boston Scientific, Medtronic, Sanofi-Aventis, and St. Jude Medical; investigator fees from Abbott, Bard, Bayer, Biosense Webster, Cameron Health, Medtronic, Pfizer, and Sanofi-Aventis; grants from Bard, Biosense Webster, Boston Scientific, ELA Sorin, Medtronic, and St. Jude Medical; and holds equity and intellectual property rights with Cameron Health. S.S., I.L.M., A.S., and M.W. are employees of Bayer AG, which provided funding for this study. At the time this study was conducted, M.W. was employed at Global Medical Affairs, Bayer AG, Berlin, Germany. She is now employed at Global Clinical Development, Bayer AG, Wuppertal, Germany.
Figures


Similar articles
-
Patient-reported treatment satisfaction and budget impact with rivaroxaban vs. standard therapy in elective cardioversion of atrial fibrillation: a post hoc analysis of the X-VeRT trial.Europace. 2016 Feb;18(2):184-90. doi: 10.1093/europace/euv294. Epub 2015 Oct 20. Europace. 2016. PMID: 26487668 Free PMC article. Clinical Trial.
-
Rationale and design of the eXplore the efficacy and safety of once-daily oral riVaroxaban for the prEvention of caRdiovascular events in patients with nonvalvular aTrial fibrillation scheduled for cardioversion trial: A comparison of oral rivaroxaban once daily with dose-adjusted vitamin K antagonists in patients with nonvalvular atrial fibrillation undergoing elective cardioversion.Am Heart J. 2014 May;167(5):646-52. doi: 10.1016/j.ahj.2013.12.024. Epub 2014 Jan 14. Am Heart J. 2014. PMID: 24766973 Clinical Trial.
-
Rivaroxaban in atrial fibrillation cardioversion: insights from the X-VeRT trial.Future Cardiol. 2015 Mar;11(2):147-51. doi: 10.2217/fca.15.4. Future Cardiol. 2015. PMID: 25760874 Clinical Trial.
-
Non-vitamin K antagonist oral anticoagulants in cardiovascular disease management: evidence and unanswered questions.J Clin Pharm Ther. 2014 Apr;39(2):118-35. doi: 10.1111/jcpt.12122. Epub 2014 Jan 3. J Clin Pharm Ther. 2014. PMID: 24383983 Review.
-
Contemporary Management of Direct Oral Anticoagulants During Cardioversion and Ablation for Nonvalvular Atrial Fibrillation.Pharmacotherapy. 2019 Jan;39(1):94-108. doi: 10.1002/phar.2205. Epub 2019 Jan 11. Pharmacotherapy. 2019. PMID: 30548542 Review.
Cited by
-
Increased Prevalence of Elevated D-Dimer Levels in Patients on Direct Oral Anticoagulants: Results of a Large Retrospective Study.Front Cardiovasc Med. 2022 Mar 31;9:830010. doi: 10.3389/fcvm.2022.830010. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35433891 Free PMC article.
-
The role of coagulome in the tumor immune microenvironment.Adv Drug Deliv Rev. 2023 Sep;200:115027. doi: 10.1016/j.addr.2023.115027. Epub 2023 Jul 28. Adv Drug Deliv Rev. 2023. PMID: 37517779 Free PMC article. Review.
-
Rivaroxaban in patients with abdominal aortic aneurysm and high-sensitivity C-reactive protein elevation (BANBOO): study protocol for a randomized, controlled trial.Trials. 2023 Jun 19;24(1):419. doi: 10.1186/s13063-023-07461-3. Trials. 2023. PMID: 37337298 Free PMC article.
-
Inflammasome Signaling, Thromboinflammation, and Venous Thromboembolism.JACC Basic Transl Sci. 2023 Jun 7;8(9):1245-1261. doi: 10.1016/j.jacbts.2023.03.017. eCollection 2023 Sep. JACC Basic Transl Sci. 2023. PMID: 37791298 Free PMC article. Review.
-
Immunomodulatory Effect of Rivaroxaban Nanoparticles Alone and in Combination with Sitagliptin on Diabetic Rat Model.Diseases. 2025 Mar 19;13(3):87. doi: 10.3390/diseases13030087. Diseases. 2025. PMID: 40136627 Free PMC article.
References
-
- Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44(11):3103–3108. - PubMed
-
- Airaksinen K EJ, Grönberg T, Nuotio I et al.Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62(13):1187–1192. - PubMed
-
- Hansen M L, Jepsen R MHG, Olesen J B et al.Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17(01):18–23. - PubMed
-
- Kirchhof P, Benussi S, Kotecha D et al.2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

