Dysregulation of Amyloid Precursor Protein Impairs Adipose Tissue Mitochondrial Function and Promotes Obesity
- PMID: 31984308
- PMCID: PMC6980705
- DOI: 10.1038/s42255-019-0149-1
Dysregulation of Amyloid Precursor Protein Impairs Adipose Tissue Mitochondrial Function and Promotes Obesity
Abstract
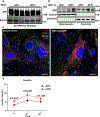
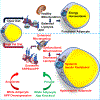
Mitochondrial function in white adipose tissue (WAT) is an important yet understudied aspect in adipocyte biology. Here, we report a role for amyloid precursor protein (APP) in compromising WAT mitochondrial function through a high-fat diet (HFD)-induced, unconventional mis-localization to mitochondria that further promotes obesity. In humans and mice, obese conditions significantly induce APP production in WAT and its enrichment in mitochondria. Mechanistically, a HFD-induced dysregulation of signal recognition particle subunit 54c is responsible for the mis-targeting of APP to adipocyte mitochondria. Mis-localized APP blocks the protein import machinery, leading to mitochondrial dysfunction in WAT. Adipocyte-specific and mitochondria-targeted APP overexpressing mice display increased body mass and reduced insulin sensitivity, along with dysfunctional WAT due to a dramatic hypertrophic program in adipocytes. Elimination of adipocyte APP rescues HFD-impaired mitochondrial function with significant protection from weight gain and systemic metabolic deficiency. Our data highlights an important role of APP in modulating WAT mitochondrial function and obesity-associated metabolic dysfunction.
Conflict of interest statement
Competing interests All the authors declare no competing interests.
Figures
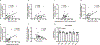
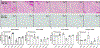



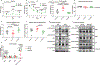






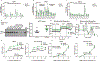
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

