Child outcomes after amnioinfusion compared with no intervention in women with second-trimester rupture of membranes: a long-term follow-up study of the PROMEXIL-III trial
- PMID: 31984652
- PMCID: PMC7818451
- DOI: 10.1111/1471-0528.16115
Child outcomes after amnioinfusion compared with no intervention in women with second-trimester rupture of membranes: a long-term follow-up study of the PROMEXIL-III trial
Abstract
Objective: To assess the effect of transabdominal amnioinfusion or no intervention on long-term outcomes in children born after second-trimester prelabour rupture of the membranes (PROM between 16+0/7 -24+0/7 weeks) and oligohydramnios.
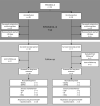
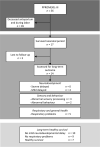
Population: Follow up of infants of women who participated in the randomised controlled trial: PPROMEXIL-III (NTR3492).
Methods: Surviving infants were invited for neurodevelopmental assessment up to 5 years of corrected age using a Bayley Scales of Infant and Toddler Development or a Wechsler Preschool and Primary Scale of Intelligence. Parents were asked to complete several questionnaires.
Main outcome measures: Neurodevelopmental outcomes were measured. Mild delay was defined as -1 standard deviation (SD), severe delay as -2 SD. Healthy long-term survival was defined as survival without neurodevelopmental delay or respiratory problems.
Results: In the amnioinfusion group, 18/28 children (64%) died versus 21/28 (75%) in the no intervention group (relative risk 0.86; 95% confidence interval [CI] 0.60-1.22). Follow-up data were obtained from 14/17 (82%) children (10 amnioinfusion, 4 no intervention). In both groups, 2/28 (7.1%) had a mild neurodevelopmental delay. No severe delay was seen. Healthy long-term survival occurred in 5/28 children (17.9%) after amnioinfusion versus 2/28 (7.1%) after no intervention (odds ratio 2.50; 95% CI 0.53-11.83). When analysing data for all assessed survivors, 10/14 (71.4%) survived without mild neurodevelopmental delay and 7/14 (50%) were classified healthy long-term survivor.
Conclusions: In this small sample of women suffering second-trimester PROM and oligohydramnios, amnioinfusion did not improve long-term outcomes. Overall, 71% of survivors had no neurodevelopmental delay.
Tweetable abstract: Healthy long-term survival was comparable for children born after second-trimester PROM and treatment with amnioinfusion or no intervention.
Keywords: Follow up; infant development; neurodevelopment; oligohydramnios; second-trimester prelabour rupture of the membranes.
© 2020 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists.
Figures
Comment in
-
Abandon ship?BJOG. 2021 Jan;128(2):302. doi: 10.1111/1471-0528.16187. Epub 2020 Mar 12. BJOG. 2021. PMID: 32115855 No abstract available.
References
-
- Williams O, Michel B, Hutchings G, Debauche C, Hubinont C. Two‐year neonatal outcome following PPROM prior to 25 weeks with a prolonged period of oligohydramnios. Early Hum Dev 2012;88:657–61. - PubMed
-
- Nourse CB, Steer PA. Perinatal outcome following conservative management of mid‐trimester pre‐labour rupture of the membranes. J Paediatr Child Health 1997;33:125–30. - PubMed
-
- Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005;32:411–28. - PubMed
-
- Hibbard JU, Hibbard MC, Ismail M, Arendt E. Pregnancy outcome after expectant management of premature rupture of the membranes in the second trimester. J Reprod Med 1993;38:945–51. - PubMed
-
- Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Degree of oligohydramnios and pregnancy outcome in patients with premature rupture of the membranes. Obstet Gynecol 1985;66:162–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources