Redox-Annulations of Cyclic Amines with ortho-Cyanomethylbenzaldehydes
- PMID: 31984752
- PMCID: PMC7104785
- DOI: 10.1021/acs.orglett.9b04506
Redox-Annulations of Cyclic Amines with ortho-Cyanomethylbenzaldehydes
Abstract
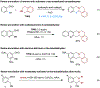
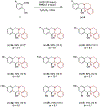
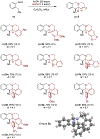
Amines such as 1,2,3,4-tetrahydroisoquinoline undergo redox-neutral annulations with ortho-cyanomethylbenzaldehydes. These amine α-C-H bond functionalization reactions are promoted by acetic acid. The resulting β-aminonitriles can be converted to the corresponding β-aminoalcohols in diastereoselective fashion.
Figures
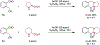
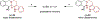

References
-
-
Selected recent reviews on amine C–H functionalization, including redox-neutral approaches:Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles.Campos KR Chem. Soc. Rev 2007, 36, 1069–1084;Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)-H Activation.Jazzar; Hitce; Renaudat A; Sofack-Kreutzer J; Baudoin O Chem. Eur. J 2010, 16, 2654–2672;Catalytic Dehydrogenative Cross-Coupling: Forming Carbon-Carbon Bonds by Oxidizing Two Carbon-Hydrogen Bonds.Yeung CS; Dong VM Chem. Rev 2011, 111, 1215–1292;Direct alpha-Functionalization of Saturated Cyclic Amines.Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW Chem. Eur. J 2012, 18, 10092–10142;Oxidative Coupling of Tertiary Amines: Scope, Mechanism and Challenges.Jones KM; Klussmann M Synlett 2012, 23, 159–162;The Redox-Neutral Approach to C-H Functionalization.Peng; Maulide Chem. Eur. J 2013, 19, 13274–13287;The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C-C Bond Formations.Girard SA; Knauber T; Li C-J Angew. Chem. Int. Ed 2014, 53, 74–100;C-H Bond Functionalization through Intramolecular Hydride Transfer.Haibach MC; Seidel D Angew. Chem. Int. Ed 2014, 53, 5010–5036;Advancement in Cascade [1,n]-Hydrogen Transfer/Cyclization: A Method for Direct Functionalization of Inactive C(sp3)-H Bonds.Wang L; Xiao J Adv. Synth. Catal 2014, 356, 1137–1171;Synthesis of Saturated N-Heterocycles.Vo C-VT; Bode JW J. Org. Chem 2014, 79, 2809–2815;The redox-A3 reaction.Seidel D Org. Chem. Front 2014, 1, 426–429;l) Catalytic asymmetric α-C(sp3)–H functionalization of amines.Qin Y; Lv J; Luo S Tetrahedron Lett 2014, 55, 551–558;The Azomethine Ylide Route to Amine C–H Functionalization: Redox-Versions of Classic Reactions and a Pathway to New Transformations.Seidel D Acc. Chem. Res 2015, 48, 317–328;Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis.Beatty JW; Stephenson CRJ Acc. Chem. Res 2015, 48, 1474–1484;Classical-Reaction-Driven Stereo- and Regioselective C(sp3)–H Functionalization of Aliphatic Amines.Mahato S; Jana CK Chem. Rec 2016, 16, 1477–1488;Organocatalysis in Inert C–H Bond Functionalization.Qin Y; Zhu L; Luo S Chem. Rev 2017, 117, 9433–9520;Recent Advances in the Enantioselective Oxidative α-C–H Functionalization of Amines.Cheng M-X; Yang S-D Synlett 2017, 28, 159–174;Transition metal-catalyzed α-alkylation of amines by C(sp3)‒H bond activation.Gonnard L; Guérinot A; Cossy J Tetrahedron 2019, 75, 145–163;Construction of N-Heterocycles through Cyclization of Tertiary Amines.Liu S; Zhao Z; Wang Y Chem. Eur. J 2019, 25, 2423–2441;Transition Metal-Catalyzed Directed C(sp3)–H Functionalization of Saturated Heterocycles.Antermite D; Bull JA Synthesis 2019, 51, 3171–3204.
-
-
-
Recent examples of mechanistically distinct amine C–H bond functionalization reactions:Palladium-Catalyzed Enantioselective C–H Activation of Aliphatic Amines Using Chiral Anionic BINOL-Phosphoric Acid Ligands.Smalley AP; Cuthbertson JD; Gaunt MJ J. Am. Chem. Soc 2017, 139, 1412–1415;Synthesis of Ring-Fused 1-Benzazepines via [1,5]-Hydride Shift/7-Endo Cyclization Sequences.Suh CW; Kwon SJ; Kim DY Org. Lett 2017, 19, 1334–1337;Direct Intermolecular C–H Functionalization Triggered by 1,5-Hydride Shift: Access to N-Arylprolinamides via Ugi-Type Reaction.Zhen L; Wang J; Xu Q-L; Sun H; Wen X; Wang G Org. Lett 2017, 19, 1566–1569;Synthesis of Spirooxindoles via the tert-Amino Effect.Ramakumar K; Maji T; Partridge JJ; Tunge JA Org. Lett 2017, 19, 4014–4017;Construction of the tetrahydroquinoline spiro skeleton via cascade [1,5]-hydride transfer-involved C(sp3)-H functionalization “on water”.Zhu S; Chen C; Xiao M; Yu L; Wang L; Xiao J Green Chem 2017, 19, 5653–5658;Organocatalytic C(sp3)–H Functionalization via Carbocation-Initiated Cascade [1,5]-Hydride Transfer/Cyclization: Synthesis of Dihydrodibenzo[b,e]azepines.Li S-S; Zhou L; Wang L; Zhao H; Yu L; Xiao J Org. Lett 2018, 20, 138–141;Direct α-C–H bond functionalization of unprotected cyclic amines.Chen W; Ma L; Paul A; Seidel D Nat. Chem 2018, 10, 165;Intramolecular hydride transfer onto arynes: redox-neutral and transition metal-free C(sp3)-H functionalization of amines.Idiris FIM; Majeste CE; Craven GB; Jones CR Chem. Sci 2018, 9, 2873–2878;Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol.Li S-S; Lv X; Ren D; Shao C-L; Liu Q; Xiao J Chem. Sci 2018, 9, 8253–8259;Second-Generation Palladium Catalyst System for Transannular C–H Functionalization of Azabicycloalkanes.Cabrera PJ; Lee M; Sanford MS J. Am. Chem. Soc 2018, 140, 5599–5606;Chiral Magnesium Bisphosphate-Catalyzed Asymmetric Double C(sp3)–H Bond Functionalization Based on Sequential Hydride Shift/Cyclization Process.Mori K; Isogai R; Kamei Y; Yamanaka M; Akiyama T J. Am. Chem. Soc 2018, 140, 6203–6207;C–H Functionalization of Amines via Alkene-Derived Nucleophiles through Cooperative Action of Chiral and Achiral Lewis Acid Catalysts: Applications in Enantioselective Synthesis.Shang M; Chan JZ; Cao M; Chang Y; Wang Q; Cook B; Torker S; Wasa M J. Am. Chem. Soc 2018, 140, 10593–10601;Electrochemical Aminoxyl-Mediated α-Cyanation of Secondary Piperidines for Pharmaceutical Building Block Diversification.Lennox AJJ; Goes SL; Webster MP; Koolman HF; Djuric SW; Stahl SS J. Am. Chem. Soc 2018, 140, 11227–11231;Borane-Catalyzed Synthesis of Quinolines Bearing Tetrasubstituted Stereocenters by Hydride Abstraction-Induced Electrocyclization.Maier AFG; Tussing S; Zhu H; Wicker G; Tzvetkova P; Flörke U; Daniliuc CG; Grimme S; Paradies J Chem. Eur. J 2018, 24, 16287–16291;Expeditious synthesis of multisubstituted indoles via multiple hydrogen transfers.Yoshida T; Mori K Chem. Commun 2018, 54, 12686–12689;B(C6F5)3-Catalyzed C–H Alkylation of N-Alkylamines Using Silicon Enolates without External Oxidant.Chan JZ; Chang Y; Wasa M Org. Lett 2019, 21, 984–988;Redox-Neutral β-C(sp3)–H Functionalization of Cyclic Amines via Intermolecular Hydride Transfer.Zhou L; Shen Y-B; An X-D; Li X-J; Li S-S; Liu Q; Xiao J Org. Lett 2019, 21, 8543–8547;Primary α-tertiary amine synthesis via α-C–H functionalization.Vasu D; Fuentes de Arriba AL; Leitch JA; de Gombert A; Dixon DJ Chem. Sci 2019, 10, 3401–3407;Deoxygenative Insertion of Carbonyl Carbon into a C(sp3)–H Bond: Synthesis of Indolines and Indoles.Asako S; Ishihara S; Hirata K; Takai K J. Am. Chem. Soc 2019, 141, 9832–9836;A General Acid-Mediated Hydroaminomethylation of Unactivated Alkenes and Alkynes.Kaiser D; Tona V; Gonçalves CR; Shaaban S; Oppedisano A; Maulide N Angew. Chem. Int. Ed 2019, 58, 14639–14643.
-
-
- alpha-Amination of Nitrogen Heterocycles: Ring-Fused Aminals.Zhang C; De CK; Mal R; Seidel D J. Am. Chem. Soc 2008, 130, 416–417; - PubMed
- A Cascade Reaction with Iminium Ion Isomerization as the Key Step Leading to Tetrahydropyrimido[4,5-d]pyrimidines.Zheng L; Yang F; Dang Q; Bai X Org. Lett 2008, 10, 889–892; - PubMed
- Metal-Free α-Amination of Secondary Amines: Computational and Experimental Evidence for Azaquinone Methide and Azomethine Ylide Intermediates.Dieckmann A; Richers MT; Platonova AY; Zhang C; Seidel D; Houk KN J. Org. Chem 2013, 78, 4132–4144; - PMC - PubMed
- Facile Access to Ring-Fused Aminals via Direct alpha-Amination of Secondary Amines with o-Aminobenzaldehydes: Synthesis of Vasicine, Deoxyvasicine, Deoxyvasicinone, Mackinazolinone, and Ruteacarpine.Richers MT; Deb I; Platonova AY; Zhang C; Seidel D Synthesis 2013, 45, 1730–1748. - PMC - PubMed
-
- Redox-Neutral α-Oxygenation of Amines: Reaction Development and Elucidation of the Mechanism.Richers MT; Breugst M; Platonova AY; Ullrich A; Dieckmann A; Houk KN; Seidel D J. Am. Chem. Soc 2014, 136, 6123–6135; - PMC - PubMed
- Redox-Neutral α-Sulfenylation of Secondary Amines: Ring-Fused N,S-Acetals.Jarvis CL; Richers MT; Breugst M; Houk KN; Seidel D Org. Lett 2014, 16, 3556–3559; - PMC - PubMed
- Divergent reaction: metal & oxidant free direct C-H aryloxylation and hydride free formal reductive N-benzylation of N-heterocycles.Mahato S; Haque MA; Dwari S; Jana CK RSC Adv 2014, 4, 46214–46217.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

