MicroRNA‑137 suppresses the proliferation, migration and invasion of cholangiocarcinoma cells by targeting WNT2B
- PMID: 31985024
- PMCID: PMC7015134
- DOI: 10.3892/ijmm.2020.4474
MicroRNA‑137 suppresses the proliferation, migration and invasion of cholangiocarcinoma cells by targeting WNT2B
Abstract
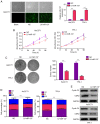
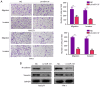
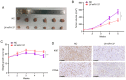
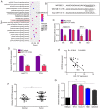
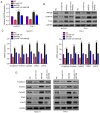
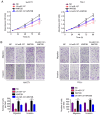
It is widely known that abnormal regulation of microRNAs (miRNAs/miRs) may contribute to the occurrence or development of tumors. The objective of the present study was to elucidate the function and underlying mechanism of miR‑137 in the progression of cholangiocarcinoma (CCA). The expression levels of miR‑137 in CCA tissues and cell lines were measured using reverse transcription‑quantitative PCR. The role of miR‑137 in the proliferation of CCA cells was assessed using the Cell Counting Kit‑8 assay, colony formation assay and cell cycle distribution analysis, while its effects on the migration and invasion of CCA cells were evaluated using Transwell assays. The function of miR‑137 on CCA growth in vivo was also investigated using a xenograft mouse model. Furthermore, the association between miR‑137 and Wnt family member 2B (WNT2B) was analyzed using bioinformatics, double luciferase assay and western blotting. It was verified that the expression of miR‑137 was low in CCA tissues and cell lines, whereas increased expression of miR‑137 significantly suppressed cell proliferation, decreased colony formation ability and induced G1 phase arrest. miR‑137 overexpression suppressed the migration and invasion ability of TFK‑1 and HuCCT1 cells. Furthermore, the results of the xenograft mouse model assays revealed that miR‑137 overexpression decreased tumor growth in vivo. The results of bioinformatics analysis and dual luciferase reporter assays demonstrated that WNT2B is directly regulated by miR‑137. The expression of WNT2B and Wnt‑pathway‑related proteins was decreased when miR‑137 was overexpressed. Restoring the expression of WNT2B notably reversed the inhibitory effect of miR‑137 on CCA cells. Therefore, the findings of the present study demonstrated that miR‑137 acts as a suppressor in CCA and inhibits CCA cell proliferation, migration and invasion through suppressing the expression of WNT2B.
Figures

References
-
- Ma WJ, Wu ZR, Shrestha A, Yang Q, Hu HJ, Wang JK, Liu F, Zhou RX, Li QS, Li FY. Effectiveness of additional resection of the invasive cancer-positive proximal bile duct margin in cases of hilar cholangiocarcinoma. Hepatobiliary Surg Nutr. 2018;7:251–269. doi: 10.21037/hbsn.2018.03.14. - DOI - PMC - PubMed

