Cyclometalated Ruthenium Pincer Complexes as Catalysts for the α-Alkylation of Ketones with Alcohols
- PMID: 31985105
- PMCID: PMC7317879
- DOI: 10.1002/chem.202000396
Cyclometalated Ruthenium Pincer Complexes as Catalysts for the α-Alkylation of Ketones with Alcohols
Abstract
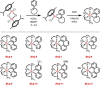
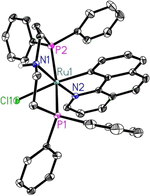
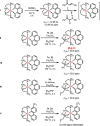
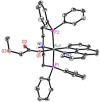
Ruthenium PNP pincer complexes bearing supplementary cyclometalated C,N-bound ligands have been prepared and fully characterized for the first time. By replacing CO and H- as ancillary ligands in such complexes, additional electronic and steric modifications of this topical class of catalysts are possible. The advantages of the new catalysts are demonstrated in the general α-alkylation of ketones with alcohols following a hydrogen autotransfer protocol. Herein, various aliphatic and benzylic alcohols were applied as green alkylating agents for ketones bearing aromatic, heteroaromatic or aliphatic substituents as well as cyclic ones. Mechanistic investigations revealed that during catalysis, Ru carboxylate complexes are predominantly formed whereas neither the PNP nor the CN ligand are released from the catalyst in significant amounts.
Keywords: C−C bond formation; hydrogen autotransfer; metallacycles; pincer complexes; ruthenium.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Manganese-Catalyzed Hydrogen-Autotransfer C-C Bond Formation: α-Alkylation of Ketones with Primary Alcohols.Angew Chem Int Ed Engl. 2016 Nov 21;55(48):14967-14971. doi: 10.1002/anie.201607072. Epub 2016 Oct 27. Angew Chem Int Ed Engl. 2016. PMID: 27785889
-
General and Mild Cobalt-Catalyzed C-Alkylation of Unactivated Amides and Esters with Alcohols.J Am Chem Soc. 2016 Aug 31;138(34):10786-9. doi: 10.1021/jacs.6b06448. Epub 2016 Aug 16. J Am Chem Soc. 2016. PMID: 27490682
-
NNN Pincer Ru(II)-Complex-Catalyzed α-Alkylation of Ketones with Alcohols.J Org Chem. 2018 Apr 6;83(7):3657-3668. doi: 10.1021/acs.joc.8b00013. Epub 2018 Mar 19. J Org Chem. 2018. PMID: 29533663
-
C-Alkylation of Ketones and Related Compounds by Alcohols: Transition-Metal-Catalyzed Dehydrogenation.Angew Chem Int Ed Engl. 2016 Jan 18;55(3):862-75. doi: 10.1002/anie.201507521. Epub 2015 Dec 7. Angew Chem Int Ed Engl. 2016. PMID: 26639633 Review.
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
Cited by
-
Complementing Pyridine-2,6-bis(oxazoline) with Cyclometalated N-Heterocyclic Carbene for Asymmetric Ruthenium Catalysis.Angew Chem Int Ed Engl. 2020 Jul 20;59(30):12392-12395. doi: 10.1002/anie.202004243. Epub 2020 Jun 5. Angew Chem Int Ed Engl. 2020. PMID: 32394593 Free PMC article.
-
Cyclometalated and NNN Terpyridine Ruthenium Photocatalysts and Their Cytotoxic Activity.Molecules. 2024 May 5;29(9):2146. doi: 10.3390/molecules29092146. Molecules. 2024. PMID: 38731639 Free PMC article.
-
Ruthenium-Catalyzed Deuteration of Aromatic Carbonyl Compounds with a Catalytic Transient Directing Group.Chemistry. 2021 Jul 7;27(38):9768-9773. doi: 10.1002/chem.202100468. Epub 2021 Jun 14. Chemistry. 2021. PMID: 33844338 Free PMC article.
-
Ligand-Promoted [Pd]-Catalyzed α-Alkylation of Ketones through a Borrowing-Hydrogen Approach.ChemistryOpen. 2023 Jan;12(1):e202200245. doi: 10.1002/open.202200245. ChemistryOpen. 2023. PMID: 36592045 Free PMC article.
References
-
- Werkmeister S., Neumann J., Junge K., Beller M., Chem. Eur. J. 2015, 21, 12226–12250. - PubMed
-
- None
-
- Peris E., Crabtree R. H., Chem. Soc. Rev. 2018, 47, 1959–1968; - PubMed
-
- Lawrence M. A. W., Green K.-A., Nelson P. N., Lorraine S. C., Polyhedron 2018, 143, 11–27;
-
- Valdés H., García-Eleno M. A., Canseco-Gonzalez D., Morales-Morales D., ChemCatChem 2018, 10, 3136–3172;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

