Constitutive accessibility of circulating proteins to hippocampal neurons in physiologically normal rats
- PMID: 31985144
- PMCID: PMC7066366
- DOI: 10.1002/brb3.1544
Constitutive accessibility of circulating proteins to hippocampal neurons in physiologically normal rats
Abstract
Introduction: Although the hippocampus (HIP) is thought impermeable to blood-borne proteins because of the integrity of the blood-brain barrier (BBB), it was recently suggested to be susceptible to hydrophilic hormones. The present study determined the accessibility of blood-borne signal molecules such as hormones to hippocampal neurons in physiologically normal rats.
Methods: As a probe for accessibility, Evans blue dye (EB) that rapidly binds to albumin (Alb), which is impermeable to the BBB, was injected intravenously. To increase the vascular permeability of the BBB, a daily single administration of angiotensin II (Ang II) was applied intravenously for seven consecutive days.
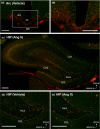
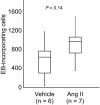
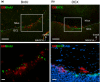
Results: Fifteen minutes after the injection of EB, histological observation revealed that a number of neurons had entrapped and accumulated EB into their cell bodies in the hippocampal dentate gyrus in all rats. Of these, relatively large oval neurons (>15 µm) in the hilus and molecular layer showed parvalbumin immunopositivity, indicating they are GABAergic interneurons. The population of EB-accumulating neurons (approximately 10 µm) were localized in the inner margin of the granule cell layer, suggesting they were granule cells. However, the number of EB-positive neurons did not change in rats treated with Ang II compared with vehicle injection.
Conclusions: These findings suggest an intriguing possibility that blood-derived proteins such as hormones have access to hippocampal neurons constitutively in the absence of stimuli that increase the vascular permeability of the BBB in a physiologically normal state.
Keywords: Evans blue dye; albumin; blood-brain barrier; granule cell layer; hilus.
© 2020 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Conflict of interest statement
Dr. Mukuda, Dr. Koyama, Dr. Nakane, and Prof. Kaidoh declare no conflicts of interest.
Figures




References
-
- Biancardi, V. C. , Son, S. J. , Ahmadi, S. , Filosa, J. A. , & Stern, J. E. (2014). Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood‐brain barrier. Hypertension, 63, 572–579. 10.1161/HYPERTENSIONAHA.113.01743 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

