Drought stress modulates cuticular wax composition of the grape berry
- PMID: 31985780
- PMCID: PMC7260727
- DOI: 10.1093/jxb/eraa046
Drought stress modulates cuticular wax composition of the grape berry
Abstract
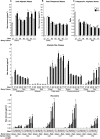
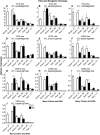
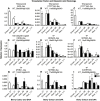
Drought events are a major challenge for many horticultural crops, including grapes, which are often cultivated in dry and warm climates. It is not understood how the cuticle contributes to the grape berry response to water deficit (WD); furthermore, the cuticular waxes and the related biosynthetic pathways are poorly characterized in this fruit. In this study, we identified candidate wax-related genes from the grapevine genome by phylogenetic and transcriptomic analyses. Developmental and stress response expression patterns of these candidates were characterized across pre-existing RNA sequencing data sets and confirmed a high responsiveness of the pathway to environmental stresses. We then characterized the developmental and WD-induced changes in berry cuticular wax composition, and quantified differences in berry transpiration. Cuticular aliphatic wax content was modulated during development and an increase was observed under WD, with wax esters being strongly up-regulated. These compositional changes were related to up-regulated candidate genes of the aliphatic wax biosynthetic pathway, including CER10, CER2, CER3, CER1, CER4, and WSD1. The effect of WD on berry transpiration was not significant. This study indicates that changes in cuticular wax amount and composition are part of the metabolic response of the grape berry to WD, but these changes do not reduce berry transpiration.
Keywords: Vitis vinifera (grapevine); Cuticle; fruit; transpiration; triterpenoids; water deficit; wax esters.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Agudelo-Romero P, Erban A, Rego C, Carbonell-Bejerano P, Nascimento T, Sousa L, Martínez-Zapater JM, Kopka J, Fortes AM. 2015. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. Journal of Experimental Botany 66, 1769–1785. - PMC - PubMed
-
- Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J. 2012. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell 24, 3106–3118. - PMC - PubMed
-
- Bernard A, Joubès J. 2013. Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Progress in Lipid Research 52, 110–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

