Ruxolitinib for the prevention of thrombosis in polycythemia vera: a systematic review and meta-analysis
- PMID: 31985808
- PMCID: PMC6988397
- DOI: 10.1182/bloodadvances.2019001158
Ruxolitinib for the prevention of thrombosis in polycythemia vera: a systematic review and meta-analysis
Abstract
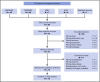
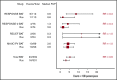
Ruxolitinib is a recommended second-line treatment for the prevention of thrombosis in patients with polycythemia vera who become resistant or intolerant to hydroxyurea; however, evidence regarding its efficacy in terms of thrombosis reduction is uncertain. We searched Medline, Embase, and archives of abstracts from the European Hematology Association and the American Society of Hematology annual congresses from 2014 onward for randomized controlled trials comparing the treatment vs best available therapy (BAT). Our search retrieved 80 records; after screening of abstracts and full text, the total was reduced to 16. Evidence came from 4 randomized controlled trials, including 663 patients (1057 patients per year). We estimated a thrombosis risk ratio of 0.56 for ruxolitinib BAT, corresponding to an incidence of 3.09% and 5.51% patients per year, respectively. The number of thrombotic events reported with ruxolitinib was consistently lower than that with BAT in our sample, but, globally, the difference did not reach significance (P = .098). Hard evidence in favor of ruxolitinib is lacking; a clinical trial on selected patients at high risk of thrombosis would be warranted, but its feasibility is questionable.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: T.B. is a member of the advisory board and has received research grant funding from Novartis and AOP (Orphan Drug Company). The remaining authors declare no competing financial interests.
Figures
References
-
- Spivak JL. How I treat polycythemia vera. Blood. 2019;134(4):341-352. - PubMed
-
- Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34(1):17-23. - PubMed
-
- Barbui T, Vannucchi AM, Finazzi G, et al. . A reappraisal of the benefit-risk profile of hydroxyurea in polycythemia vera: a propensity-matched study. Am J Hematol. 2017;92(11):1131-1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical