Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants
- PMID: 31985831
- PMCID: PMC6984659
- DOI: 10.1002/14651858.CD010061.pub4
Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants
Update in
-
Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants.Cochrane Database Syst Rev. 2022 Dec 15;12(12):CD010061. doi: 10.1002/14651858.CD010061.pub5. Cochrane Database Syst Rev. 2022. PMID: 36519620 Free PMC article.
Abstract
Background: In preterm newborns, the ductus arteriosus frequently fails to close and the infants require medical or surgical closure of the patent ductus arteriosus (PDA). A PDA can be treated surgically; or medically with one of two prostaglandin inhibitors, indomethacin or ibuprofen. Case reports suggest that paracetamol may be an alternative for the closure of a PDA. An association between prenatal or postnatal exposure to paracetamol and later development of autism or autism spectrum disorder has been reported.
Objectives: To determine the effectiveness and safety of intravenous or oral paracetamol compared with placebo or no intervention, intravenous indomethacin, intravenous or oral ibuprofen, or with other cyclo-oxygenase inhibitors for treatment of an echocardiographically diagnosed PDA in preterm or low birth weight infants.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 10), MEDLINE via PubMed (1966 to 6 November 2017), Embase (1980 to 6 November 2017), and CINAHL (1982 to 6 November 2017). We searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCT) and quasi-randomised trials.
Selection criteria: We included RCTs in which paracetamol was compared to no intervention, placebo or other agents used for closure of PDA irrespective of dose, duration and mode of administration in preterm (≤ 34 weeks' postmenstrual age) infants. We both reviewed the search results and made a final selection of potentially eligible articles by discussion. We included studies of both prophylactic and therapeutic use of paracetamol.
Data collection and analysis: We performed data collection and analyses in accordance with the methods of the Cochrane Neonatal Review Group. We used the GRADE approach to assess the quality of evidence for the following outcomes when data were available: failure of ductal closure after the first course of treatment; neurodevelopmental impairment; all-cause mortality during initial hospital stay (death); gastrointestinal bleed or stools positive for occult blood; and serum levels of creatinine after treatment (µmol/L).
Main results: We included eight studies that reported on 916 infants. One of these studies compared paracetamol to both ibuprofen and indomethacin. Five studies compared treatment of PDA with paracetamol versus ibuprofen and enrolled 559 infants. There was no significant difference between paracetamol and ibuprofen for failure of ductal closure after the first course of drug administration (typical risk ratio (RR) 0.95, 95% confidence interval (CI) 0.75 to 1.21; typical risk difference (RD) -0.02, 95% CI -0.09 to 0.09); I² = 0% for RR and RD; moderate quality of evidence. Four studies (n = 537) reported on gastrointestinal bleed which was lower in the paracetamol group versus the ibuprofen group (typical RR 0.28, 95% CI 0.12 to 0.69; typical RD -0.06, 95% CI -0.09 to -0.02); I² = 0% for RR and RD; number needed to treat for an additional beneficial outcome (NNTB) 17 (95% CI 11 to 50); moderate quality of evidence. The serum levels of creatinine were lower in the paracetamol group compared with the ibuprofen group in four studies (moderate quality of evidence), as were serum bilirubin levels following treatment in two studies (n = 290). Platelet counts and daily urine output were higher in the paracetamol group compared with the ibuprofen group. One study reported on long-term follow-up to 18 to 24 months of age following treatment with paracetamol versus ibuprofen. There were no significant differences in the neurological outcomes at 18 to 24 months (n = 61); (low quality of evidence). Two studies compared prophylactic administration of paracetamol for a PDA with placebo or no intervention in 80 infants. Paracetamol resulted in a lower rate of failure of ductal closure after 4 to 5 days of treatment compared to placebo or no intervention which was of borderline significance for typical RR 0.49 (95% CI 0.24 to 1.00; P = 0.05); but significant for typical RD -0.21 (95% CI -0.41 to -0.02); I² = 0 % for RR and RD; NNTB 5 (95% CI 2 to 50); (low quality of evidence). Two studies (n = 277) compared paracetamol with indomethacin. There was no significant difference in the failure to close a PDA (typical RR 0.96, 95% CI 0.55 to 1.65; I² = 11%; typical RD -0.01, 95% CI -0.09 to 0.08; I² = 17%) (low quality of evidence). Serum creatinine levels were significantly lower in the paracetamol group compared with the indomethacin group and platelet counts and daily urine output were significantly higher in the paracetamol group.
Authors' conclusions: Moderate-quality evidence according to GRADE suggests that paracetamol is as effective as ibuprofen; low-quality evidence suggests paracetamol to be more effective than placebo or no intervention; and low-quality evidence suggests paracetamol as effective as indomethacin in closing a PDA. There was no difference in neurodevelopmental outcome in children exposed to paracetamol compared to ibuprofen; however the quality of evidence is low and comes from only one study. In view of concerns raised regarding neurodevelopmental outcomes following prenatal and postnatal exposure to paracetamol, long-term follow-up to at least 18 to 24 months' postnatal age must be incorporated in any studies of paracetamol in the newborn population. At least 19 ongoing trials have been registered. Such trials are required before any recommendations for the possible routine use of paracetamol in the newborn population can be made.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Arne Ohlsson ‒ no conflict of interest to declare.
Prakeshkumar Shah ‒ no conflict of interest to declare.
Figures
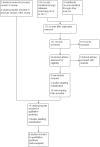
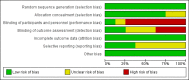


















































































Update of
-
Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD010061. doi: 10.1002/14651858.CD010061.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Jan 27;1:CD010061. doi: 10.1002/14651858.CD010061.pub4. PMID: 29624206 Free PMC article. Updated.
References
References to studies included in this review
Al‐Lawama 2017 {published data only}
Asbagh 2015 {published data only}
-
- Asbagh PA, Zarkesh MR, Nili F, Sadat Nayeri FS, Naeem AT. Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran University Medical Journal 2015;73(2):86‐92. [http://tumj.tums.ac.ir/article‐1‐6603‐en.html]
Dang 2013 {published data only}
Dash 2015 {published data only}
-
- Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or intravenous indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatrics 2015;52(7):573‐8. [PUBMED: 26244949] - PubMed
-
- Kabra NS, Dash SK. Comparison of enteral paracetamol and intravenous indomethacin in closure of patent ductus arteriosus (PDA) in preterm newborns: A randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2014 07 17‐18; Vienna, Austria. 2014.
El Mashad 2017 {published data only}
-
- El‐Mashad AE, El‐Mahdy H, Amrousy DE, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. European Journal of Pediatrics 2017;176(2):233‐40. [DOI: 10.1007/s00431-016-2830-7; PUBMED: 28004188] - DOI - PubMed
Härkin 2016 {published data only}
Oncel 2014 {published data only}
-
- Oncel MY, Eras Z, Uras N, Canpolat FC, Erdeve O, Oguz SS. Neurodevelopmental outcomes of preterm infants treated with oral paracetamol versus ibuprofen for patent ductus arteriosus. American Journal of Perinatology 2017;34(12):1185–9. - PubMed
-
- Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: A randomized controlled trial. Journal of Pediatrics 2014;164(3):510‐4.e1. [PUBMED: 24359938] - PubMed
Yang 2016 {published data only}
References to studies awaiting assessment
Babaei 2018 {published data only}
-
- Babaei H, Nemati R, Daryoshi H. Closure of patent ductus arteriosus with oral acetaminophen in preterm neonates: A randomized trial. Biomedical Research and Therapy 2018;5(2):2034‐44.
Bagheri 2016 {published data only}
Kluckow 2016 {published data only}
-
- Kluckow MR, Carlisle H, Broom M, Woods P, Jeffery M, Desai D, et al. A randomised blinded placebo controlled trial of paracetamol to treat later PDA. Journal of Paediatrics and Child Health 2016;52(Suppl 2):100.
References to ongoing studies
ACTRN12613000289718 {published data only}
-
- ACTRN12613000289718. Paracetamol for patent ductus arteriosus treatment: comparison between oral and intravenous administration. Australian New Zealand Clinical Trials Registry (first received 8 March 2013).
ACTRN12616001517460 {published data only}
-
- ACTRN12616001517460. Early paracetamol (EPAR) to promote early closure of the ductus arteriosus in preterm infants. Australian New Zealand Clinical Trials Registry (first received 1 November 2016).
ChiCTR‐TRC‐13003912 {published data only}
-
- ChiCTR‐TRC‐13003912. Comparison of oral paracetamol versus ibuprofen in premature infants<1500g with patent ductus arteriosus: A randomized controlled trial. Chinese Clinical Trial Register (ChiCTR) (first received 7 December 2013).
CTRI/2016/09/007261 {published data only}
-
- CTRI/2016/09/007261. Oral ibuprofen versus paracetamol on ductus arteriosus [Comparison of oral paracetamol versus ibuprofen for PDA closure in preterms ‐ a randomized controlled single blinded study ‐ IPOD]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/09/007261 (first received 8 September 2016).
CTRI/2017/10/009989 {published data only}
-
- CTRI/2017/10/009989. Paracetamol versus ibuprofen for closure of patent ductus arteriosus [Efficacy and safety of oral paracetamol versus oral ibuprofen in management of patent ductus arteriosus in preterm neonates less than or equal to 34 Weeks or less than or equal to 1800 gms: a randomized control trial ‐ BAP trial]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/009989 (first received 10 October 2017).
CTRI/2017/10/010012 {published data only}
-
- CTRI/2017/10/010012. A clinical trial comparing low dose versus standard dose of intravenous paracetamol for PDA closure in very premature babies [Randomized controlled trial of two different doses of intravenous paracetamol for PDA closure in preterm infants less than 30 weeks]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/010012 (first received 05 October 2017).
EUCTR2013‐003883‐30‐IT {published data only}
-
- EUCTR2013‐003883‐30‐IT. Efficacy and safety of paracetamol in the treatment of patent ductus arteriosus in preterm infants [Efficacy and safety of paracetamol in comparison to ibuprofen for patent ductus arteriosus treatment in preterm infants. A randomized, open label, comparator‐controlled, prospective study. ‐ Paracetamol in Patent Ductus Arteriosus]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐003883‐30‐IT (first received 12 November 2013).
EUCTR2015‐003177‐14‐ES {published data only}
-
- EUCTR2015‐003177‐14‐ES. Paracetamol versus ibuprofen in preterm infants with a hemodynamically significant patent ductus arteriosus: a randomized clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐003177‐14‐ES (first received 20 April 2016).
IRCT2016081729404N1 {published data only}
-
- IRCT2016081729404N1. Safety and efficacy of venous paracetamol with venous ibubrofen in treatment of patent ductus arteriosus (PDA) among premature neonates hospitalized in NICU, Zanjan Ayatollah Musavi Hospital in 2016‐ 2017. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016081729404N1 (first received 9 May 2017).
Kumar 2017 {published data only}
-
- Kumar A, Sundaram V, Yadav R, Oleti TP, Murki S, Krishna A. Oral paracetamol versus oral ibuprofen for closure of haemodynamically significant patent ductus arteriosus in preterm neonates (<32 weeks): a blinded, randomised, active‐controlled, non‐inferiority trial. BMJ Paediatrics Open 2017;1(1):e000143. [DOI: 10.1136/bmjpo-2017-000143; CTRI/2014/08/004805] - DOI - PMC - PubMed
NCT01291654 {published data only}
-
- NCT01291654. Paracetamol and patent ductus arteriosus (PDA) [Paracetamol in the treatment of patent ductus arteriosus in the premature neonate]. Clinicaltrials.gov/show/NCT01291654 (first received 6 February 2011).
NCT01938261 {published data only}
-
- NCT01938261. The preterm infants' paracetamol study (PreParaS). Clinicaltrials.gov/show/NCT01938261 (first received 10 September 2013).
NCT02002741 {published data only}
-
- NCT02002741. Adding paracetamol to ibuprofen for treatment of patent ductus arteriosus in preterm infants [Adding paracetamol to ibuprofen for treatment of patent ductus arteriosus in preterm infants: a pilot, double blind, randomized, placebo‐control trial]. ClinicalTrials.gov/show/NCT02002741 (first received 6 December 2013).
NCT02056223 {published data only}
-
- NCT02056223. Paracetamol vs ibuprofen for PDA closure in preterm infants. (PARIDA) [Paracetamol versus ibuprofen for patent ductus arteriosus closure in preterm infants. A prospective, randomized, controlled, double blind, multicenter clinical trial]. clinicaltrials.gov/show/NCT02056223 (first received 10 August 2005).
NCT02422966 {published data only}
-
- NCT02422966. Paracetamol in Patent Ductus Arteriosus [Efficacy and safety of paracetamol in comparison to ibuprofen for Patent Ductus Arteriosus treatment in preterm infants: A randomized, open label, comparator‐controlled, prospective study]. clinicaltrials.gov/show/NCT02422966 (first received 22 April 2015).
NCT02819414 {published data only}
-
- NCT02819414. Paracetamol treatment of the borderline significant PDA [Time to re‐evaluate the kinder gentler approach to patent ductus arteriosus (PDA) in the preterm neonate]. clinicaltrials.gov/show/NCT02819414 (first received 30 June 2016).
NCT03008876 {published data only}
-
- NCT03008876. IV acetaminophen and patent ductus arteriosus [The efficacy of IV acetaminophen on patent ductus arteriosus closure in preterm infants]. clinicaltrials.gov/show/NCT03008876 (first received 4 January 2017).
NCT03103022 {published data only}
-
- NCT03103022. Combination of acetaminophen and ibuprofen in the management of patent ductus arteriosus in premature infants: a pilot study. clinicaltrials.gov/show/NCT03103022 (first received 06 April 2017).
NCT03265782 {published data only}
-
- NCT03265782. Paracetamol versus ibuprofen for PDA closure [Comparison between the effect of oral paracetamol versus oral ibuprofen in the treatment of patent ductus arteriosus in preterm and low birth weight infants]. clinicaltrials.gov/show/NCT03265782 (first received 29 August 2017).
Additional references
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Association, 2013.
Arana 2001
-
- Arana A, Morton NS, Hansen TG. Treatment with paracetamol in infants. Acta Anaesthesiologica Scandinavica 2001;45(1):20‐9. [PUBMED: 11152028] - PubMed
Avella‐Garcia 2016
-
- Avella‐Garcia CB, Julvez J, Fortuny J, Rebordosa C, García‐Esteban R, Galán IR, et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. International Journal of Epidemiology 2016;45(6):1987‐96. [DOI: 10.1093/ije/dyw115; PUBMED: 27353198] - DOI - PubMed
Bauer 2013
Bauer 2018
Bell 1978
Bornehag 2017
Botting 2000
Clyman 2000
Das 2014
-
- Das RR, Arora K, Naik SS. Efficacy and safety of paracetamol versus ibuprofen for treating patent ductus arteriosus in preterm infants: A meta‑analysis. Journal of Clinical Neonatology 2014;3(4):183‐90.
Edwards 1990
-
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, et al. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 1990;335(8704):1491‐5. [PUBMED: 1972434] - PubMed
Ehrenkranz 2005
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353‐60. [DOI: 10.1542/peds.2005-0249; PUBMED: 16322158] - DOI - PubMed
El‐Khuffash 2014
Fowlie 2010
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 21 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grèen 1989
-
- Grèen K, Drvota V, Vesterqvist O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989;37(3):311‐5. [PUBMED: 2664901] - PubMed
Hammerman 1995
-
- Hammerman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clinics in Perinatology 1995;22(2):457‐79. [PUBMED: 7671547] - PubMed
Hammerman 2011
-
- Hammerman C, Bin‐Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 2011;128(6):e1618‐21. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Huang 2017
-
- Huang X, Wang F, Wang K. Paracetamol versus ibuprofen for the treatment of patent ductus arteriosus in preterm neonates: a meta‐analysis of randomized controlled trials. Journal of Maternal‐fetal & Neonatal Medicine 2017 Jul 18 [Epub ahead of print]. [DOI: 10.1080/14767058.2017.1338263; PUBMED: 28720053] - DOI - PubMed
ICCROP 2005
Jobe 2001
Kessel 2014
-
- Kessel I, Waisman D, Lavie‐Nevo K, Golzman M, Lorber A, Rotschild A. Paracetamol effectiveness, safety and blood level monitoring during patent ductus arteriosus closure: a case series. Journal of Maternal‐fetal & Neonatal Medicine 2014;27(16):1719‐21. [DOI: 10.3109/14767058.2013.871630; PUBMED: 24460433] - DOI - PubMed
Lee 2000
-
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network 1996‐1997. Pediatrics 2000;106(5):1070‐9. [PUBMED: 11061777] - PubMed
Maisels 2003
Mathew 1998
-
- Mathew R. Development of the pulmonary circulation: metabolic aspects. In: Polin RA, Fox WW editor(s). Fetal and Neonatal Physiology. Vol. 1, Philadelphia: W.B. Saunders, 1998:924‐9.
Mitra 2016
-
- Mitra S, Florez ID, Tamayo ME, Aune D, Mbuagbaw L, Veroniki AA, et al. Effectiveness and safety of treatments used for the management of patent ductus arteriosus (PDA) in preterm infants: a protocol for a systematic review and network meta‐analysis. BMJ Open 2016;6(7):e011271. [DOI: 10.1136/bmjopen-2016-011271; PUBMED: 27456327] - DOI - PMC - PubMed
Nadir 2014
Naulty 1978
-
- Naulty CM, Horn S, Conry J, Avery GB. Improved lung compliance after ligation of patent ductus arteriosus in hyaline membrane disease. Journal of Pediatrics 1978;93(4):682‐4. [PUBMED: 702251] - PubMed
NLM 2012
-
- U.S. National Library of Medicine. Drug Information Portal. druginfo.nlm.nih.gov/drugportal (accessed 21 March 2018).
Ohlsson 1993
-
- Ohlsson A, Bottu J, Govan J, Ryan ML, Fong K, Myhr T. Effect of indomethacin on cerebral blood flow velocities in very low birth weight neonates with patent ductus arteriosus. Developmental Pharmacology and Therapeutics 1993;20(1‐2):100‐6. [PUBMED: 7924757] - PubMed
Ohlsson 2011
Ohlsson 2015a
Palmer 2008
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Peterson 1985
-
- Peterson RG. Consequences associated with nonnarcotic analgesics in the fetus and newborn. Federation Proceedings 1985;44(7):2309‐13. [PUBMED: 3884385] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sakhalkar 1992
-
- Sakhalkar VS, Merchant RH. Therapy of symptomatic patent ductus arteriosus in preterms with mefenemic acid and indomethacin. Indian Pediatrics 1992;29(3):313‐8. [PUBMED: 1612672] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seyberth 1983
-
- Seyberth HW, Rascher W, Hackenthal R, Wille L. Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very‐low‐birth‐weight infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1993;103(6):979‐84. [PUBMED: 6358443] - PubMed
Simbi 2002
Sinah 2013
Sundaram 2011
Terrin 2014
Terrin 2016
-
- Terrin G, Conte F, Oncel MY, Scipione A, McNamara PJ, Simons S, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta‐analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(2):F127‐36. [DOI: 10.1136/archdischild-2014-307312; PUBMED: 26283668] - DOI - PubMed
Van Loon 2011
-
- Loon RL, Roofthooft MT, Hillege HL, Harkel AD, Osch‐Gevers M, Delhaas T, et al. Pediatric pulmonary hypertension in the Netherlands, Epidemiology and characterization during the period 1991 to 2005. Circulation 2011;124(16):1755‐64. [DOI: 10.1161/CIRCULATIONAHA.110.969584; PUBMED: 21947294] - DOI - PubMed
Viberg 2014
-
- Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicological Sciences 2014;138(1):139‐47. [DOI: 10.1093/toxsci/kft329; PUBMED: 24361869] - DOI - PubMed
Walls 2007
Weir 1999
-
- Weir FJ, Ohlsson A, Myhr TL, Fong K, Ryan ML. A patent ductus arteriosus is associated with reduced middle cerebral artery blood flow velocity. European Journal of Pediatrics 1999;158(6):484‐7. [PUBMED: 10378397] - PubMed
Wolf 1989
-
- Wolf WM, Snover DC, Leonard AS. Localized intestinal perforation following intravenous indomethacin in premature infants. Journal of Pediatric Surgery 1989;24(4):409‐10. [PUBMED: 2732888] - PubMed
Ystrom 2017
Yurttutan 2013
-
- Yurttutan S, Oncel MY, Arayici S, Uras N, Altug N, Erdeve O, et al. A different first‐choice drug in the medical management of patent ductus arteriosus: oral paracetamol. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(8):825‐7. [DOI: 10.3109/14767058.2012.755162; PUBMED: 23205872] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

