Optogenetic mapping of feeding and self-stimulation within the lateral hypothalamus of the rat
- PMID: 31986148
- PMCID: PMC6984703
- DOI: 10.1371/journal.pone.0224301
Optogenetic mapping of feeding and self-stimulation within the lateral hypothalamus of the rat
Abstract
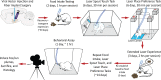
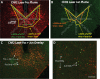
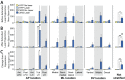
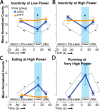
The lateral hypothalamus (LH) includes several anatomical subregions involved in eating and reward motivation. This study explored localization of function across different LH subregions in controlling food intake stimulated by optogenetic channelrhodopsin excitation, and in supporting laser self-stimulation. We particularly compared the tuberal LH subregion, the posterior LH subregion, and the lateral preoptic area. Local diameters of tissue optogenetically stimulated within the LH were assessed by measuring laser-induced Fos plumes and Jun plumes via immunofluorescence surrounding optic fiber tips. Those plume diameters were used to map localization of function for behavioral effects elicited by LH optogenetic stimulation. Optogenetic stimulation of the tuberal subsection of the LH produced the most robust eating behavior and food intake initially, but produced only mild laser self-stimulation in the same rats. However, after repeated exposures to optogenetic stimulation, tuberal LH behavioral profiles shifted toward more self-stimulation and less food intake. By contrast, stimulation of the lateral preoptic area produced relatively little food intake or self-stimulation, either initially or after extended stimulation experience. Stimulation in the posterior LH subregion supported moderate self-stimulation, but not food intake, and at higher laser intensity shifted valence to evoke escape behaviors. We conclude that the tuberal LH subregion may best mediate stimulation-bound increases in food intake stimulated by optogenetic excitation. However, incentive motivational effects of tuberal LH stimulation may shift toward self-stimulation behavior after repeated stimulation. By contrast, the lateral preoptic area and posterior LH do not as readily elicit either eating behavior or laser self-stimulation, and may be more prone to higher-intensity aversive effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures











Similar articles
-
Lateral Hypothalamic GABAergic Neurons Encode Reward Predictions that Are Relayed to the Ventral Tegmental Area to Regulate Learning.Curr Biol. 2017 Jul 24;27(14):2089-2100.e5. doi: 10.1016/j.cub.2017.06.024. Epub 2017 Jul 6. Curr Biol. 2017. PMID: 28690111 Free PMC article.
-
Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA.J Neurosci. 2016 Mar 9;36(10):2975-85. doi: 10.1523/JNEUROSCI.3799-15.2016. J Neurosci. 2016. PMID: 26961951 Free PMC article.
-
Optogenetic Activation of a Lateral Hypothalamic-Ventral Tegmental Drive-Reward Pathway.PLoS One. 2016 Jul 7;11(7):e0158885. doi: 10.1371/journal.pone.0158885. eCollection 2016. PLoS One. 2016. PMID: 27387668 Free PMC article.
-
Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake.Physiol Behav. 2011 Jul 25;104(1):40-6. doi: 10.1016/j.physbeh.2011.04.046. Epub 2011 May 4. Physiol Behav. 2011. PMID: 21550353 Review.
-
Hypothalamic self-stimulation and stimulation escape in relation to feeding and mating.Fed Proc. 1979 Oct;38(11):2454-61. Fed Proc. 1979. PMID: 385351 Review.
Cited by
-
Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging.Brain Sci. 2022 Mar 24;12(4):431. doi: 10.3390/brainsci12040431. Brain Sci. 2022. PMID: 35447963 Free PMC article. Review.
-
Introducing the PLOS ONE Collection on the neuroscience of reward and decision making.PLoS One. 2020 Oct 8;15(10):e0240505. doi: 10.1371/journal.pone.0240505. eCollection 2020. PLoS One. 2020. PMID: 33031482 Free PMC article.
-
Topographic organization of bidirectional connections between the cingulate region (infralimbic area and anterior cingulate area, dorsal part) and the interbrain (diencephalon) of the adult male rat.bioRxiv [Preprint]. 2024 Oct 1:2024.09.29.615708. doi: 10.1101/2024.09.29.615708. bioRxiv. 2024. PMID: 40093037 Free PMC article. Preprint.
-
Chronic water restriction reduces sensitivity to brain stimulation reward in male and female rats.Physiol Behav. 2023 May 1;263:114110. doi: 10.1016/j.physbeh.2023.114110. Epub 2023 Feb 3. Physiol Behav. 2023. PMID: 36740136 Free PMC article.
-
Activation of neurons in the insular cortex and lateral hypothalamus during food anticipatory period caused by food restriction in mice.J Physiol Sci. 2023 Dec 8;73(1):34. doi: 10.1186/s12576-023-00892-2. J Physiol Sci. 2023. PMID: 38066417 Free PMC article.
References
-
- Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000. July 24;423(2):261–81. - PubMed
-
- Herrick CJ. The morphology of the forebrain in amphibia and reptilia. J Comp Neurol Psychol. 20(5):413–547.
-
- Krieg WJS. The hypothalamus of the albino rat. J Comp Neurol. 1932;55(1):19–89.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

