Membrane conductances of mouse cone photoreceptors
- PMID: 31986199
- PMCID: PMC7054858
- DOI: 10.1085/jgp.201912520
Membrane conductances of mouse cone photoreceptors
Abstract
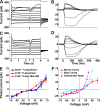
Vertebrate photoreceptor cells respond to light through a closure of CNG channels located in the outer segment. Multiple voltage-sensitive channels in the photoreceptor inner segment serve to transform and transmit the light-induced outer-segment current response. Despite extensive studies in lower vertebrates, we do not know how these channels produce the photoresponse of mammalian photoreceptors. Here we examined these ionic conductances recorded from single mouse cones in unlabeled, dark-adapted retinal slices. First, we show measurements of the voltage dependence of the light response. After block of voltage-gated Ca2+ channels, the light-dependent current was nearly linear within the physiological range of voltages with constant chord conductance and a reversal potential similar to that previously determined in lower vertebrate photoreceptors. At a dark resting membrane potential of -45 mV, cones maintain a standing Ca2+ current (iCa) between 15 and 20 pA. We characterized the time and voltage dependence of iCa and a calcium-activated anion channel. After constitutive closure of the CNG channels by the nonhydrolysable analogue GTP-γ-S, we observed a light-dependent increase in iCa followed by a Ca2+-activated K+ current, both probably the result of feedback from horizontal cells. We also recorded the hyperpolarization-activated cyclic nucleotide-gated (HCN) conductance (ih) and measured its current-voltage relationship and reversal potential. With small hyperpolarizations, ih activated with a time constant of 25 ms; activation was speeded with larger hyperpolarizations. Finally, we characterized two voltage-gated K+-conductances (iK). Depolarizing steps beginning at -10 mV activated a transient, outwardly rectifying iK blocked by 4-AP and insensitive to TEA. A sustained iK isolated through subtraction was blocked by TEA but was insensitive to 4-AP. The sustained iK had a nearly linear voltage dependence throughout the physiological voltage range of the cone. Together these data constitute the first comprehensive study of the channel conductances of mouse photoreceptors.
© 2020 Ingram et al.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

