Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
- PMID: 31986871
- PMCID: PMC7093283
- DOI: 10.4132/jptm.2019.11.06
Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
Abstract
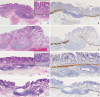
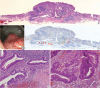
Background: Colorectal epithelial neoplasm extending into the submucosal gut-associated lymphoid tissue (GALT) can cause difficulties in the differential diagnosis. Regarding GALT-associated epithelial neoplasms, a few studies favor the term "GALT carcinoma" while other studies have mentioned the term "GALT-associated pseudoinvasion/epithelial misplacement (PEM)".
Methods: The clinicopathologic characteristics of 11 cases of colorectal epithelial neoplasm associated with submucosal GALT diagnosed via endoscopic submucosal dissection were studied.
Results: Eight cases (72.7%) were in males. The median age was 59 years, and age ranged from 53 to 73. All cases had a submucosal tumor component more compatible with GALT-associated PEM. Eight cases (72.7%) were located in the right colon. Ten cases (90.9%) had a non-protruding endoscopic appearance. Nine cases (81.8%) showed continuity between the submucosal and surface adenomatous components. Nine cases showed (81.8%) focal defects or discontinuation of the muscularis mucosae adjacent to the submucosal GALT. No case showed hemosiderin deposits in the submucosa or desmoplastic reaction. No case showed single tumor cells or small clusters of tumor cells in the submucosal GALT. Seven cases (63.6%) showed goblet cells in the submucosa. No cases showed oncocytic columnar cells lining submucosal glands.
Conclusions: Our experience suggests that pathologists should be aware of the differential diagnosis of GALT-associated submucosal extension by colorectal adenomatous neoplasm. Further studies are needed to validate classification of GALT-associated epithelial neoplasms.
Keywords: Adenomatous polyps; Colorectal neoplasms; Humans; Lymphoid tissue.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

