Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway
- PMID: 31988091
- PMCID: PMC7044461
- DOI: 10.1242/bio.046854
Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway
Erratum in
-
Correction: Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway.Biol Open. 2023 Jan 1;12(1):bio059765. doi: 10.1242/bio.059765. Epub 2023 Jan 12. Biol Open. 2023. PMID: 36634210 Free PMC article. No abstract available.
Abstract
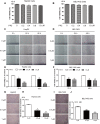
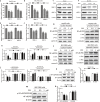
The morbidity and mortality of primary liver cancer is one of the highest amongst all cancers. Deficiency of effective treatment and characteristics of cancer metastasis are believed to be responsible for this situation, thus a great demand is required for new agent development. Polyphyllin II (PP2), an important steroidal saponin extracted from Rhizoma Paris, has emerged as a potential anti-cancer agent, but the effects of PP2 in liver cancers and its underlying mechanisms remain unexplored. In our study, we found that PP2 could remarkably suppress the proliferation of two liver cancer cell lines, HepG2 and BEL7402, resulting in significant cell death. Besides, low doses of PP2 have displayed properties that inhibit cellular motility and invasion of liver cancer cells. In addition, we have found that PP2-mediated cofilin activity suppression was implicated in the inhibition of liver cancer cell motility. Decreased expression of two major hydrolytic enzymes (MMP2/MMP9), through the AKT/NF-κB signaling pathway may also be also responsible for this process. Rescue experiments done with either non-phosphorylatable mutant cofilin-1 (S3A) transfection or an activator of the AKT pathway significantly reversed the inhibition effects of PP2 on liver cancer cells. Taken together, we report a potential agent for liver cancer treatment and reveal its underlying mechanisms.
Keywords: AKT/NF-κB; Cofilin; Liver cancer; MMP2/MMP9; Migration and invasion; Polyphyllin II; Proliferation.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Chen J.-S., Huang X.-H., Wang Q., Huang J.-Q., Zhang L.-J., Chen X.-L., Lei J. and Cheng Z.-X. (2013). Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis 34, 10-19. 10.1093/carcin/bgs274 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

