Optimization of a Plasmodium falciparum circumsporozoite protein repeat vaccine using the tobacco mosaic virus platform
- PMID: 31988134
- PMCID: PMC7022184
- DOI: 10.1073/pnas.1911792117
Optimization of a Plasmodium falciparum circumsporozoite protein repeat vaccine using the tobacco mosaic virus platform
Abstract
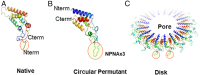
Plasmodium falciparum vaccine RTS,S/AS01 is based on the major NPNA repeat and the C-terminal region of the circumsporozoite protein (CSP). RTS,S-induced NPNA-specific antibody titer and avidity have been associated with high-level protection in naïve subjects, but efficacy and longevity in target populations is relatively low. In an effort to improve upon RTS,S, a minimal repeat-only, epitope-focused, protective, malaria vaccine was designed. Repeat antigen copy number and flexibility was optimized using the tobacco mosaic virus (TMV) display platform. Comparing antigenicity of TMV displaying 3 to 20 copies of NPNA revealed that low copy number can reduce the abundance of low-affinity monoclonal antibody (mAb) epitopes while retaining high-affinity mAb epitopes. TMV presentation improved titer and avidity of repeat-specific Abs compared to a nearly full-length protein vaccine (FL-CSP). NPNAx5 antigen displayed as a loop on the TMV particle was found to be most optimal and its efficacy could be further augmented by combination with a human-use adjuvant ALFQ that contains immune-stimulators. These data were confirmed in rhesus macaques where a low dose of TMV-NPNAx5 elicited Abs that persisted at functional levels for up to 11 mo. We show here a complex association between NPNA copy number, flexibility, antigenicity, immunogenicity, and efficacy of CSP-based vaccines. We hypothesize that designing minimal epitope CSP vaccines could confer better and more durable protection against malaria. Preclinical data presented here supports the evaluation of TMV-NPNAx5/ALFQ in human trials.
Keywords: CSP; antigenicity; immunogenicity; malaria; vaccines.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: M.D.L., F.A.K., A.H.B., and S.D. have filed a patent on the tobacco mosaic virus (TMV)-based malaria vaccine described herein.
Figures


References
-
- World Health Organiztion , World Malaria Report 2018 (WHO, Geneva, 2018).
-
- Lockyer M. J., Schwarz R. T., Strain variation in the circumsporozoite protein gene of Plasmodium falciparum. Mol. Biochem. Parasitol. 22, 101–108 (1987). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

