Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo
- PMID: 31988501
- PMCID: PMC7147967
- DOI: 10.1038/s41565-019-0626-4
Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo
Abstract
Human epidermal growth factor receptor 2 (HER2) is overexpressed in >20% of breast cancers. Dimerization of HER2 receptors leads to the activation of downstream signals enabling the proliferation and survival of malignant phenotypes. Owing to the high expression levels of HER2, combination therapies are currently required for the treatment of HER2+ breast cancer. Here, we designed non-toxic transformable peptides that self-assemble into micelles under aqueous conditions but, on binding to HER2 on cancer cells, transform into nanofibrils that disrupt HER2 dimerization and subsequent downstream signalling events leading to apoptosis of cancer cells. The phase transformation of peptides enables specific HER2 targeting, and inhibition of HER2 dimerization blocks the expression of proliferation and survival genes in the nucleus. We demonstrate, in mouse xenofraft models, that these transformable peptides can be used as a monotherapy in the treatment of HER2+ breast cancer.
Conflict of interest statement
Competing interests
The authors declare the following competing financial interest(s): K.S.L., L.Z., D.J. and L.W. are the co-inventors of a pending patent on the fibrillar transformable nanoparticles. K.S.L. is the founding scientist of LamnoTherapeutics Inc. which plans to develop the nanotherapeutics described in the manuscript. The remaining authors declare no competing interests.
Figures




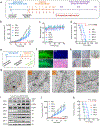
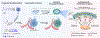
References
-
- Slamon DJ et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987). - PubMed
-
- Arteaga CL et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat. Rev. Clin. Oncol 9, 16–32 (2011). - PubMed
-
- Gravalos C & Jimeno A HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann. Oncol 19, 1523–1529 (2008). - PubMed
-
- Ferte C, Andre F & Soria JC Molecular circuits of solid tumours: prognostic and predictive tools for bedside use. Nat. Rev. Clin. Oncol 7, 367–380 (2010). - PubMed
-
- Roukos DH & Briasoulis E Individualized preventive and therapeutic management of hereditary breast ovarian cancer syndrome. Nat. Clin. Pract. Oncol 4, 578–590 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

