Effect of the micro-environment on α-synuclein conversion and implication in seeded conversion assays
- PMID: 31988747
- PMCID: PMC6966864
- DOI: 10.1186/s40035-019-0181-9
Effect of the micro-environment on α-synuclein conversion and implication in seeded conversion assays
Abstract
Background: α-Synuclein is a small soluble protein, whose physiological function in the healthy brain is poorly understood. Intracellular inclusions of α-synuclein, referred to as Lewy bodies (LBs), are pathological hallmarks of α-synucleinopathies, such as Parkinson's disease (PD) or dementia with Lewy bodies (DLB).
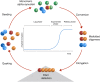
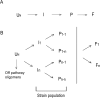
Main body: Understanding of the molecular basis as well as the factors or conditions promoting α-synuclein misfolding and aggregation is an important step towards the comprehension of pathological mechanism of α-synucleinopathies and for the development of efficient therapeutic strategies. Based on the conversion and aggregation mechanism of α-synuclein, novel diagnostic tests, such as protein misfolding seeded conversion assays, e.g. the real-time quaking-induced conversion (RT-QuIC), had been developed. In diagnostics, α-synuclein RT-QuIC exhibits a specificity between 82 and 100% while the sensitivity varies between 70 and 100% among different laboratories. In addition, the α-synuclein RT-QuIC can be used to study the α-synuclein-seeding-characteristics of different α-synucleinopathies and to differentiate between DLB and PD.
Conclusion: The variable diagnostic accuracy of current α-synuclein RT-QuIC occurs due to different protocols, cohorts and material etc.. An impact of micro-environmental factors on the α-synuclein aggregation and conversion process and the occurrence and detection of differential misfolded α-synuclein types or strains might underpin the clinical heterogeneity of α-synucleinopathies.
Keywords: Protein misfolding; Protein strains; RT-QuIC; α-Synucleinopathies.
© The Author(s). 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

