Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials
- PMID: 31988978
- PMCID: PMC6970133
- DOI: 10.1016/j.omtm.2019.11.018
Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials
Abstract
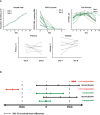
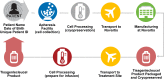
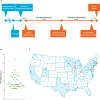
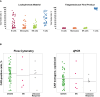
Tisagenlecleucel is a CD19-specific chimeric antigen receptor (CAR)-T cell therapy approved for patients aged ≤25 years with relapsed or refractory B cell precursor acute lymphoblastic leukemia (B-ALL) and adults with relapsed or refractory diffuse large B cell lymphoma (DLBCL). The initial tisagenlecleucel manufacturing process technology was developed at an academic center and was subsequently transferred, optimized, validated, and scaled out to supply large global trials before commercialization. Tisagenlecleucel manufactured in two centralized facilities has been successfully used in global multicenter trials for B-ALL and DLBCL (>50 clinical centers in 12 countries). In this paper, we describe some of the continuous process improvements made to tisagenlecleucel manufacturing over time to meet global demand while maintaining and improving product quality. During early tisagenlecleucel clinical trials, process enhancements were made to address logistical challenges related to manufacturing for multicenter trials and to accommodate the variability observed in patient starting cellular material. These enhancements resulted in improvements in manufacturing capacity, process robustness, manufacturing success rates, and product quality, and reductions in throughput time. In summary, through continuous evaluation and improvements based on experience during global trials, a consistent and robust commercial manufacturing process for tisagenlecleucel has been developed, leading to improvements in manufacturing success when compared to the initial processes.
Keywords: chimeric antigen receptor-T cell therapy; commercial manufacturing; global manufacturing; immunotherapy; manufacturing process optimization.
© 2019 The Authors.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

