Microfluidic hemophilia models using blood from healthy donors
- PMID: 31989085
- PMCID: PMC6971334
- DOI: 10.1002/rth2.12286
Microfluidic hemophilia models using blood from healthy donors
Abstract
Background: Microfluidic clotting assays permit drug action studies for hemophilia therapeutics under flow. However, limited availability of patient samples and Inter-donor variability limit the application of such assays, especially with many patients on prophylaxis.
Objective: To develop approaches to phenocopy hemophilia using modified healthy blood in microfluidic assays.
Methods: Corn trypsin inhibitor (4 µg/mL)-treated healthy blood was dosed with either anti-factor VIII (FVIII; hemophilia A model) or a recombinant factor IX (FIX) missense variant (FIX-V181T; hemophilia B model). Treated blood was perfused at 100 s-1 wall shear rate over collagen/tissue factor (TF) or collagen/factor XIa (FXIa).
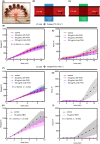
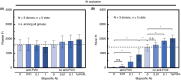
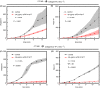
Results: Anti-FVIII partially blocked fibrin production on collagen/TF, but completely blocked fibrin production on collagen/FXIa, a phenotype reversed with 1 µmol/L bispecific antibody (emicizumab), which binds FIXa and factor X. As expected, emicizumab had no significant effect on healthy blood (no anti-FVIII present) perfused over collagen/FXIa. The efficacy of emicizumab in anti-FVIII-treated healthy blood phenocopied the action of emicizumab in the blood of a patient with hemophilia A perfused over collagen/FXIa. Interestingly, a patient-derived FVIII-neutralizing antibody reduced fibrin production when added to healthy blood perfused over collagen/FXIa. For low TF surfaces, reFIX-V181T (50 µg/mL) fully blocked platelet and fibrin deposition, a phenotype fully reversed with anti-TFPI.
Conclusion: Two new microfluidic hemophilia A and B models demonstrate the potency of anti-TF pathway inhibitor, emicizumab, and a patient-derived inhibitory antibody. Using collagen/FXIa-coated surfaces resulted in reliable and highly sensitive hemophilia models.
Keywords: drug evaluation; fibrin; hemophilia; hemostasis; microfluidics.
© 2019 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals, Inc on behalf of International Society on Thrombosis and Haemostasis.
Figures



Similar articles
-
Recombinant factor VIIa addition to haemophilic blood perfused over collagen/tissue factor can sufficiently bypass the factor IXa/VIIIa defect to rescue fibrin generation.Haemophilia. 2017 Sep;23(5):759-768. doi: 10.1111/hae.13259. Epub 2017 May 5. Haemophilia. 2017. PMID: 28475272 Free PMC article.
-
A modified thrombin generation assay to evaluate the plasma coagulation potential in the presence of emicizumab, the bispecific antibody to factors IXa/X.Int J Hematol. 2020 Nov;112(5):621-630. doi: 10.1007/s12185-020-02959-x. Epub 2020 Aug 3. Int J Hematol. 2020. PMID: 32748217
-
FXIa and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow.Blood. 2015 Sep 17;126(12):1494-502. doi: 10.1182/blood-2015-04-641472. Epub 2015 Jul 1. Blood. 2015. PMID: 26136249 Free PMC article.
-
Bispecific Antibodies and Advances in Non-Gene Therapy Options in Hemophilia.Res Pract Thromb Haemost. 2020 Apr 28;4(4):446-454. doi: 10.1002/rth2.12337. eCollection 2020 May. Res Pract Thromb Haemost. 2020. PMID: 32548546 Free PMC article. Review.
-
Usefulness of chromogenic assays for potency assignment and recovery of plasma-derived FVIII and FIX concentrates or their recombinant long acting therapeutic equivalents with potential application in treated pediatric hemophiliac patients.Transfus Apher Sci. 2018 Jun;57(3):363-369. doi: 10.1016/j.transci.2018.05.020. Epub 2018 May 16. Transfus Apher Sci. 2018. PMID: 29895509 Review.
Cited by
-
Structure of Blood Coagulation Factor VIII in Complex With an Anti-C2 Domain Non-Classical, Pathogenic Antibody Inhibitor.Front Immunol. 2021 Jun 10;12:697602. doi: 10.3389/fimmu.2021.697602. eCollection 2021. Front Immunol. 2021. PMID: 34177966 Free PMC article.
-
A Synergistic Overview between Microfluidics and Numerical Research for Vascular Flow and Pathological Investigations.Sensors (Basel). 2024 Sep 10;24(18):5872. doi: 10.3390/s24185872. Sensors (Basel). 2024. PMID: 39338617 Free PMC article. Review.
References
-
- Mannucci PM, Tuddenham EGD. The hemophilias — from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. - PubMed
-
- Barnes C, Blanchette V, Lillicrap D, Mann K, Stain AM, Leggo J, et al. Different clinical phenotype in triplets with haemophilia A. Haemophilia. 2007;13:202–5. - PubMed
-
- Santagostino E, Mancuso ME, Tripodi A, Chantarangkul V, Clerici M, Garagiola IMP. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010;8:737–43. - PubMed
-
- Carcao MD, Marijke van den Berg H, Ljung RMM. Correlation between phenotype and genotype in a large unselected cohort of children with severe hemophilia A. Blood. 2013;121:3946–52. - PubMed
-
- van den Berg HM, De Groot PH, Fischer K Phenotypic heterogeneity in severe hemophilia. J Thromb Haemost. 2007;5:151–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

