Mechanisms of checkpoint inhibition-induced adverse events
- PMID: 31989585
- PMCID: PMC7160658
- DOI: 10.1111/cei.13421
Mechanisms of checkpoint inhibition-induced adverse events
Abstract
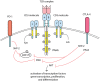
Immune checkpoint inhibition has revolutionized the treatment of several solid cancers, most notably melanoma and non-small-cell lung cancer (NSCLC). Drugs targeting cytotoxic T lymphocyte antigen (CTLA)-4 and programmed cell death 1 (PD-1) have made their way into routine clinical use; however, this has not been without difficulties. Stimulation of the immune system to target cancer has been found to result in a reduction of self-tolerance, leading to the development of adverse effects that resemble autoimmunity. These adverse effects are erratic in their onset and severity and can theoretically affect any organ type. Several mechanisms for immune-related toxicity have been investigated over recent years; however, no consensus on the cause or prediction of toxicity has been reached. This review seeks to examine reported evidence for possible mechanisms of toxicity, methods for prediction of those at risk and a discussion of future prospects within the field.
Keywords: CTLA-4; PD-1; cancer; checkpoint; immunotherapy.
© 2020 British Society for Immunology.
Conflict of interest statement
S. P. has received honoraria from BMS, MSD, Roche, Amgen and GSK. N. P. has received personal fees as a speaker for Allergan, Bristol‐Myers Squibb, Falk, Janssen, Tillotts and Takeda, and as a consultant and/or an advisory board member for AbbVie, Allergan, Celgene, Ferring and Vifor Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet [internet] 2001; 357:539–45. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11229684(accessed 4 November 2019). - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity [internet] 2004; 21:137–48. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15308095 (accessed 4 November 2019). - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. - PubMed
-
- Brunet J‐F, Denizot F, Luciani M‐F et al A new member of the immunoglobulin superfamily – CTLA‐4. Nature [internet] 1987; 328:267–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3496540 (accessed 4 November 2019). - PubMed
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science [internet] 1996; 271:1734–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8596936 (accessed 4 November 2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

