Time-to-Event Modeling for Remimazolam for the Indication of Induction and Maintenance of General Anesthesia
- PMID: 31989598
- PMCID: PMC7079111
- DOI: 10.1002/jcph.1552
Time-to-Event Modeling for Remimazolam for the Indication of Induction and Maintenance of General Anesthesia
Abstract
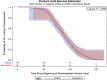
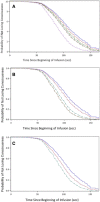
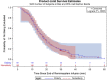
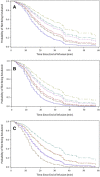
Remimazolam is an ultra-short-acting benzodiazepine being investigated for induction and maintenance of general anesthesia and for procedural sedation. This dose-response analysis of 4 phase 2-3 studies evaluated covariates that may impact the pharmacodynamic profile (based on theoretical pharmacokinetic principles) and require dose adjustments in subpopulations, particularly elderly, and if remimazolam has cumulative properties. Covariates affecting the time to loss of consciousness and time to extubation were evaluated using Cox proportional hazards models. Factors affecting steady-state infusion rate required to produce adequate sedation were evaluated using linear regression. Variability in time to loss of consciousness was explained by induction dose, age, body mass index, and time from initiation of opioids to initiation of remimazolam. The steady-state infusion rate producing adequate sedation was higher in European than Japanese subjects due to differences in study design. American Society of Anesthesiologists physical status class 3 subjects had a 28% lower maintenance infusion rate than class 1 subjects. Other statistically significant covariates (American Society of Anesthesiologists class 2, estimated glomerular filtration rate, and sex) resulted in small (≤14%), non-clinically relevant differences. Factors affecting time to extubation included the last infusion rate (ie, tapering), the bispectral index score at the end of infusion, and sex. The time to extubation after remimazolam did not increase with increased cumulative dose of remimazolam or duration of surgery. This evaluation of remimazolam's pharmacodynamic profile, in the absence of pharmacokinetic data, informed dosing recommendations and showed that remimazolam does not have cumulative properties in the general anesthesia setting.
Keywords: anesthesia; dose-response; remimazolam; sedation; time-to-event analysis.
© 2020 Paion UK Ltd. The Journal of Clinical Pharmacology published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
F.S., K.U.P., and T.S. are employees of Paion GmbH. V.S. and L.L. are paid consultants for Paion GmbH.
Figures
References
-
- Remimazolam Doi M.. Journal of Japan Society for Clinical Anesthesia. 2014;34(7):860‐866.
-
- Holford NH, Sheiner LB. Understanding the dose‐effect relationship: clinical application of pharmacokinetic‐pharmacodynamic models. Clin Pharmacokinet. 1981;6(6):429‐453. - PubMed
-
- Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d‐tubocurarine. Clin Pharmacol Ther. 1979;25(3):358‐371. - PubMed
-
- Kreuer S, Bruhn J, Larsen R, Bialas P, Wilhelm W. Comparability of Narcotrend index and bispectral index during propofol anaesthesia. Br J Anaesth. 2004;93(2):235‐240. - PubMed
-
- Platten HP, Schweizer E, Dilger K, Mikus G, Klotz U. Pharmacokinetics and the pharmacodynamic action of midazolam in young and elderly patients undergoing tooth extraction. Clin Pharmacol Ther. 1998;63(5):552‐560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

