Guanidine hydrochloride reactivates an ancient septin hetero-oligomer assembly pathway in budding yeast
- PMID: 31990274
- PMCID: PMC7056273
- DOI: 10.7554/eLife.54355
Guanidine hydrochloride reactivates an ancient septin hetero-oligomer assembly pathway in budding yeast
Abstract
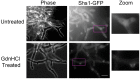
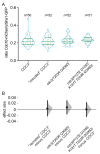
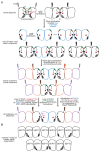
Septin proteins evolved from ancestral GTPases and co-assemble into hetero-oligomers and cytoskeletal filaments. In Saccharomyces cerevisiae, five septins comprise two species of hetero-octamers, Cdc11/Shs1-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11/Shs1. Slow GTPase activity by Cdc12 directs the choice of incorporation of Cdc11 vs Shs1, but many septins, including Cdc3, lack GTPase activity. We serendipitously discovered that guanidine hydrochloride rescues septin function in cdc10 mutants by promoting assembly of non-native Cdc11/Shs1-Cdc12-Cdc3-Cdc3-Cdc12-Cdc11/Shs1 hexamers. We provide evidence that in S. cerevisiae Cdc3 guanidinium occupies the site of a 'missing' Arg side chain found in other fungal species where (i) the Cdc3 subunit is an active GTPase and (ii) Cdc10-less hexamers natively co-exist with octamers. We propose that guanidinium reactivates a latent septin assembly pathway that was suppressed during fungal evolution in order to restrict assembly to octamers. Since homodimerization by a GTPase-active human septin also creates hexamers that exclude Cdc10-like central subunits, our new mechanistic insights likely apply throughout phylogeny.
Keywords: S. cerevisiae; biochemistry; cell biology; chaperone; chemical biology; guanidine; oligomerization; protein folding; septins.
Plain language summary
For a cell to work and perform its role, it relies on molecules called proteins that are made up of chains of amino acids. Individual proteins can join together like pieces in a puzzle to form larger, more complex structures. How the protein subunits fit together depends on their individual shapes and sizes. Many cells contain proteins called septins, which can assemble into larger protein complexes that are involved in range of cellular processes. The number of subunits within these complexes differs between organisms and sometimes even between cell types in the same organism. For example, yeast typically have eight subunits within a septin protein complex and struggle to survive when the number of septin subunits is reduced to six. Whereas other organisms, including humans, can make septin protein complexes containing six or eight subunits. However, it is poorly understood how septin proteins are able to organize themselves into these different sized complexes. Now, Johnson et al. show that a chemical called guanidinium helps yeast make complexes containing six septin subunits. Guanidinium has many similarities to the amino acid arginine. Comparing septins from different species revealed that one of the septin proteins in yeast lacks a key arginine component. This led Johnson et al. to propose that when guanidinium binds to septin at the site where arginine should be, this steers the septin protein towards the shape required to make a six-subunit complex. These findings reveal a new detail of how some species evolved complexes consisting of different numbers of subunits. This work demonstrates a key difference between complexes made up of six septin proteins and complexes which are made up of eight, which may be relevant in how different human cells adapt their septin complexes for different purposes. It may also become possible to use guanidinium to treat genetic diseases that result from the loss of arginine in certain proteins.
© 2020, Johnson et al.
Conflict of interest statement
CJ, MS, AW, AK, AG, AB, MM No competing interests declared
Figures










References
-
- Abbey M, Hakim C, Anand R, Lafera J, Schambach A, Kispert A, Taft MH, Kaever V, Kotlyarov A, Gaestel M, Menon MB. GTPase domain driven dimerization of SEPT7 is dispensable for the critical role of septins in fibroblast cytokinesis. Scientific Reports. 2016;6:20007. doi: 10.1038/srep20007. - DOI - PMC - PubMed
-
- Amberg D, Burke D, Strathern J. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 Edition. Cold Spring Harbor Laboratory Press; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

