Rapid versus slow withdrawal of antiepileptic drugs
- PMID: 31990368
- PMCID: PMC6986471
- DOI: 10.1002/14651858.CD005003.pub3
Rapid versus slow withdrawal of antiepileptic drugs
Update in
-
Rapid versus slow withdrawal of antiepileptic drugs.Cochrane Database Syst Rev. 2022 Jan 10;1(1):CD005003. doi: 10.1002/14651858.CD005003.pub4. Cochrane Database Syst Rev. 2022. PMID: 35005782 Free PMC article.
Abstract
Background: The ideal objective of treating a person with epilepsy is to induce remission (free of seizures for some time) using antiepileptic drugs (AEDs) and withdraw the AEDs without causing seizure recurrence. Prolonged usage of AEDs may have long-term adverse effects. Hence, when a person with epilepsy is in remission, it is logical to attempt to discontinue the medication. The timing of withdrawal and the mode of withdrawal arise while contemplating withdrawal of AEDs. This review examines the evidence for the rate of withdrawal of AEDs (whether rapid or slow tapering) and its effect on seizure recurrence. This is an updated version of the original Cochrane Review published in 2006, Issue 2.
Objectives: To quantify risk of seizure recurrence after rapid (tapering period of three months or less) or slow (tapering period of more than three months) discontinuation of antiepileptic drugs in adults and children with epilepsy who are in remission, and to assess which variables modify the risk of seizure recurrence.
Search methods: For the latest update, on 9 April 2019, we searched: Cochrane Register of Studies (CRS Web, which includes the Cochrane Epilepsy Group Specialized Register, CENTRAL, and ClinicalTrials.gov), MEDLINE (Ovid; 8 April 2019), the WHO International Clinical Trials Registry Platform, and SCOPUS. There were no language restrictions.
Selection criteria: Randomized controlled trials that evaluate withdrawal of AEDs in a rapid or slow tapering after varying periods of seizure control in people with epilepsy.
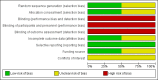
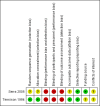
Data collection and analysis: Review authors independently assessed the trials for inclusion and extracted the data. The outcomes assessed included seizure freedom after one, two, or five years of AED withdrawal; time to recurrence of seizure following withdrawal; occurrence of status epilepticus; mortality; morbidity due to seizure, such as injuries, fractures, and aspiration pneumonia; and quality of life (assessed by validated scale).
Main results: In this review update, we have included one new study. The new study randomized 57 children with epilepsy with seizure freedom for at least two years to taper the AED during over one or six months. The study was not blinded and there were no details of randomization. Over the period of 54 months of follow-up, 20/30 participants in the one-month group remained seizure-free compared to 15/27 participants in the six-month group (no evidence of a difference). There was no information on time of seizure recurrence for each group to allow a comparison. One trial had already been included in the previous version of the review; it involved 149 children. There was a non-significant trend toward a lower risk of seizure recurrence after one year of AED withdrawal in participants allocated to slow tapering (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.58 to 1.01; P= 0.06; very low-certainty evidence). At the end of two years, 30 participants were seizure free in the rapid-tapering group and 29 participants in the slow-tapering group (RR 0.87, 95% CI 0.58 to 1.29; P = 0.48; very low-certainty evidence). At the end of five years, 10 participants were seizure free in the rapid-tapering group and six participants in the slow-tapering group (RR 1.40, 95% CI 0.54 to 3.65; P = 0.49; very low-certainty evidence). There were no data for the other outcomes. Due to the methodological heterogeneity and the difference in the duration of tapering we did not perform a quantitative synthesis of these studies.
Authors' conclusions: Since the last version of this review was published, we found one new pediatric study. In view of methodological deficiencies, and small sample size of the two included studies, we cannot draw any reliable conclusions regarding the optimal rate of tapering of AEDs. Using GRADE, we assessed the certainty of the evidence as very low for outcomes for which data were available. We judged both studies to be at high risk of bias. Further studies are needed in adults and children to investigate the optimal rate of withdrawal of AEDs and to study the effects of variables such as seizure types, etiology, mental retardation, electroencephalography abnormalities, presence of neurologic deficits, and other comorbidities on the rate of tapering.
Contexte: L'objectif idéal du traitement d'une personne épileptique est d'induire une rémission (absence de crises pendant un certain temps) en utilisant des médicaments antiépileptiques (MAE) et d’arrêter les MAE sans provoquer de récidive des crises. L'utilisation prolongée des MAE pourrait avoir des effets indésirables à long terme. Par conséquent, lorsqu'une personne épileptique est en rémission, il est logique de tenter d'interrompre la médication. La question du moment et du mode d’arrêt se posent lorsque l'on envisage l’arrêt des MAE. Cette revue examine les données probantes sur le taux d’arrêt des MAE (qu'il s'agisse d'un arrêt rapide ou lent) et son effet sur la récurrence des crises. Ceci est une version mise à jour de la revue Cochrane originale publiée en 2006, numéro 2.
Objectifs: Quantifier le risque de récidive des crises après un arrêt rapide (période de diminution progressive de trois mois ou moins) ou lent (période de diminution progressive de plus de trois mois) des médicaments antiépileptiques chez les adultes et les enfants épileptiques en rémission, et évaluer les variables qui modifient le risque de récidive des crises. STRATÉGIE DE RECHERCHE DOCUMENTAIRE: Pour la dernière mise à jour, le 9 avril 2019, nous avons fait des recherches dans : le registre Cochrane des études cliniques (CRS Web, qui comprend le registre spécialisé du groupe Cochrane sur l’épilepsie, CENTRAL, et ClinicalTrials.gov), MEDLINE (Ovid ; 8 avril 2019), le système d'enregistrement international des essais cliniques (ICTRP) de l’OMS et SCOPUS. Aucune restriction de langue n’a été appliquée. CRITÈRES DE SÉLECTION: Essais contrôlés randomisés qui évaluent l’arrêt des MAE, par une diminution rapide ou lente, après diverses périodes de contrôle des crises chez les personnes épileptiques. RECUEIL ET ANALYSE DES DONNÉES: Les auteurs de l'étude ont, de manière indépendante, évalué les essais pour l'inclusion et ont extrait les données. Les critères de jugement évalués comprenaient l’absence de crise après un, deux ou cinq ans d’arrêt des MAE ; le délai sans récidive de crise après arrêt ; l'apparition d’un état de mal épileptique ; la mortalité ; la morbidité due à la crise, comme les blessures, les fractures et la pneumonie d’aspiration ; et la qualité de vie (évaluée par une échelle validée). RÉSULTATS PRINCIPAUX: Dans cette mise à jour de la revue, nous avons inclus une nouvelle étude. La nouvelle étude a randomisé 57 enfants souffrant d'épilepsie et ne souffrant pas de crises depuis au moins deux ans afin de réduire la prise des MAE pendant au moins un à six mois. L'étude n'a pas été réalisée en aveugle et il n'y a pas eu de détails sur la randomisation. Au cours de la période de suivi de 54 mois, 20/30 participants du groupe un mois sont restés sans crise d'épilepsie, contre 15/27 participants du groupe six mois (pas de preuve de différence). Il n'y avait pas d’information sur le moment de la récidive des crises dans chaque groupe afin de permettre une comparaison. Un essai avait déjà été inclus dans la version précédente de la revue ; il concernait 149 enfants. Nous avons constaté une tendance non‐significative de diminution du risque de récidive des crises après un an d’arrêt des MAE, chez les participants affectés à la diminution lente (rapport de risque (RR) 0,76, intervalle de confiance (IC) à 95% 0,58 à 1,01 ; P= 0,06 ; données probantes de très faible certitude). Au bout de deux ans, 30 participants étaient exempts de crises dans le groupe à diminution rapide et 29 participants dans le groupe à diminution lente (RR 0,87, 95 % IC 0,58 à 1,29 ; P = 0,48 ; données probantes de très faible certitude). Au bout de cinq ans, dix participants étaient exempts de crises d'épilepsie dans le groupe à diminution rapide et six dans le groupe à diminution lente (RR 1,40, IC 95% 0,54 à 3,65 ; P = 0,49 ; données probantes de très faible certitude). Il n'y avait pas de données sur les autres critères de jugement. En raison de l'hétérogénéité méthodologique et de différence dans la durée de la diminution, nous n'avons pas effectué de synthèse quantitative de ces études.
Conclusions des auteurs: Depuis la publication de la dernière version de cette revue, nous avons trouvé une nouvelle étude pédiatrique. Compte tenu des lacunes méthodologiques et de la petite taille de l'échantillon des deux études incluses, nous ne pouvons pas tirer de conclusions fiables concernant le rythme de diminution optimal des MAE. En utilisant GRADE, nous avons évalué la certitude des données probantes comme étant très faible pour les résultats pour lesquels des données étaient disponibles. Nous avons jugé que les deux études présentaient un risque de biais élevé. D'autres études sont nécessaires chez les adultes et les enfants pour étudier le taux d’arrêt optimal des MAE et pour étudier les effets de variables telles que les types de crises, l'étiologie, le retard mental, les anomalies sur l'électroencéphalogramme, la présence de déficits neurologiques et d'autres comorbidités sur le rythme de diminution.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
FAL: none known. EGT: none known. FB: has acted as a consultant and received travel support from Eisai; he has received payment for lectures from UCB Pharma.
Figures
Comment in
-
Does rapid withdrawal of antiseizure medication increase risk of seizure recurrence in epilepsy? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2021 Aug;63(8):897-898. doi: 10.1111/dmcn.14906. Epub 2021 Apr 26. Dev Med Child Neurol. 2021. PMID: 33904198 No abstract available.
References
References to studies included in this review
Serra 2005 {published data only}
Tennison 1994 {published data only}
References to studies excluded from this review
Aidaros 2010 {published data only}
-
- Aidaros MA, Siam AG. Effect of the duration of withdrawal of antiepileptic drugs on the risk of seizure recurrence in childhood epilepsy. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2010;47(4):593‐8.
Braathen 1996 {published data only}
-
- Braathen G, Andersson T, Gylje H, Melander H, Naglo AS, Noren L, et al. Comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study. I. Outcome in different seizure types. Epilepsia 1996;37(9):822‐32. - PubMed
Gebremariam 1999 {published data only}
-
- Gebremariam A, Mengesha W, Enqusilassie F. Discontinuing anti‐epileptic medication(s) in epileptic children: 18 versus 24 months. Annals of Tropical Paediatrics 1999;19(1):93‐9. - PubMed
Gherpelli 1992 {published data only}
-
- Gherpelli JL, Kok F, dal Forno S, Elkis LC, Lefevre BH, Diament AJ. Discontinuing medication in epileptic children: a study of risk factors related to recurrence. Epilepsia 1992;33(4):681‐6. - PubMed
He 2016 {published data only}
-
- He RQ, Zeng QY, Zhu P, Bao YX, Zheng RY, Xu HQ. Risk of seizure relapse after antiepileptic drug withdrawal in adult patients with focal epilepsy. Epilepsy & Behavior 2016;64(Pt A):233‐8. - PubMed
MRC 1991 {published data only}
-
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Lancet 1991;337(8751):1175‐80. - PubMed
Peters 1998 {published data only}
-
- Peters AC, Brouwer OF, Geerts AT, Arts WF, Stroink H, Donselaar CA. Randomized prospective study of early discontinuation of antiepileptic drugs in children with epilepsy. Neurology 1998;50(3):724‐30. - PubMed
Todt 1984 {published data only}
-
- Todt H. The late prognosis of epilepsy in childhood: results of a prospective follow‐up study. Epilepsia 1984;25(2):137‐44. - PubMed
Verrotti 2000 {published data only}
-
- Verrotti A, Morresi S, Basciani F, Cutarella R, Morgese G, Chiarelli F. Discontinuation of anticonvulsant therapy in children with partial epilepsy. Neurology 2000;55(9):1393‐5. - PubMed
References to ongoing studies
Gasparini 2016 {published data only}
-
- Gasparini S, Ferlazzo E, Giussani G, Italiano D, Cianci V, Sueri C, et al. Rapid versus slow withdrawal of antiepileptic monotherapy in 2‐year seizure‐free adult patients with epilepsy (RASLOW) study: a pragmatic multicentre, prospective, randomized, controlled study. Neurological Sciences 2016;37(4):579‐83. [DOI: 10.1007/s10072-016-2483-3; PUBMED: 26809952] - DOI - PubMed
Additional references
AAN 1996
-
- American Academy of Neurology – Quality Standards Subcommittee. Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure‐free patients‐‐summary statement.. Neurology 1996;47(2):600‐2. - PubMed
Arts 1988
-
- Arts WF, Visser LH, Loonen MC, Tjiam AT, Stroink H, Stuurman PM, et al. Follow‐up of 146 children with epilepsy after withdrawal of antiepileptic therapy. Epilepsia 1988;29(3):244‐50. - PubMed
Berg 1994
-
- Berg AT, Shinnar S. Relapse following discontinuation of antiepileptic drugs: a meta‐analysis. Neurology 1994;44(4):601‐8. - PubMed
Bouma 1987
Brodie 2012
Callaghan 1988
-
- Callaghan N, Garrett A, Goggin T. Withdrawal of anticonvulsant drugs in patients free of seizures for two years: a prospective study. New England Journal of Medicine 1988;318(15):942‐6. - PubMed
Duncan 1987
-
- Duncan JS, Shorvon SD. Rates of antiepileptic drug reduction in active epilepsy – current practice. Epilepsy Research 1987;1(6):357‐64. - PubMed
Emerson 1981
-
- Emerson R, D'Souza BJ, Vining EP, Holden KR, Mellits ED, Freeman JM. Stopping medication in children with epilepsy: predictors of outcome. New England Journal of Medicine 1981;304(19):1125‐9. - PubMed
Fisher 2014
-
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55(4):475‐82. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), Accessed 16 July 2019).
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PUBMED: 18436948] - DOI - PMC - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Juul‐Jensen 1964
-
- Juul‐Jensen P. Frequency of recurrence after discontinuance of anti‐convulsant therapy in patients with epileptic seizures. Epilepsia 1964;5:352‐63. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
MRC 1993
Oller‐Daurella 1975
-
- Oller‐Daurella L, Pamies R, Oller LF. Reduction or discontinuance of antiepileptic drugs in patients seizure‐free for more than 5 years. In: Janz D editor(s). Epileptology: Proceedings of the Seventh International Symposium on Epilepsy. Berlin (Germany): Georg Thieme Verlag, 1975:218‐27.
Overweg 1981
-
- Overweg J, Rowan AJ, Binnie CD, Oosting J, Nagelkerke NJ. Prediction of seizure recurrence after withdrawal of antiepileptic drugs. In: Dam M, Gram L, Penry JK editor(s). Advances in Epileptology: the XIIth Epilepsy International Symposium. New York: Raven Press, 1981:503‐8.
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shinnar 1994
-
- Shinnar S, Berg AT, Moshe SL, Kang H, O'Dell C, Alemany M, et al. Discontinuing antiepileptic drugs in children with epilepsy: a prospective study. Annals of Neurology 1994;35(5):534‐45. - PubMed
References to other published versions of this review
Ranganathan 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous