T-495, a novel low cooperative M1 receptor positive allosteric modulator, improves memory deficits associated with cholinergic dysfunction and is characterized by low gastrointestinal side effect risk
- PMID: 31990455
- PMCID: PMC6986443
- DOI: 10.1002/prp2.560
T-495, a novel low cooperative M1 receptor positive allosteric modulator, improves memory deficits associated with cholinergic dysfunction and is characterized by low gastrointestinal side effect risk
Abstract
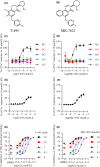
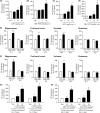
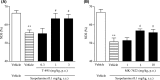
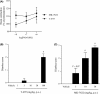
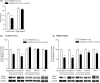
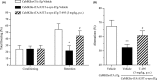
M1 muscarinic acetylcholine receptor (M1 R) activation can be a new therapeutic approach for the treatment of cognitive deficits associated with cholinergic hypofunction. However, M1 R activation causes gastrointestinal (GI) side effects in animals. We previously found that an M1 R positive allosteric modulator (PAM) with lower cooperativity (α-value) has a limited impact on ileum contraction and can produce a wider margin between cognitive improvement and GI side effects. In fact, TAK-071, a novel M1 R PAM with low cooperativity (α-value of 199), improved scopolamine-induced cognitive deficits with a wider margin against GI side effects than a high cooperative M1 R PAM, T-662 (α-value of 1786), in rats. Here, we describe the pharmacological characteristics of a novel low cooperative M1 R PAM T-495 (α-value of 170), using the clinically tested higher cooperative M1 R PAM MK-7622 (α-value of 511) as a control. In rats, T-495 caused diarrhea at a 100-fold higher dose than that required for the improvement of scopolamine-induced memory deficits. Contrastingly, MK-7622 showed memory improvement and induction of diarrhea at an equal dose. Combination of T-495, but not of MK-7622, and donepezil at each sub-effective dose improved scopolamine-induced memory deficits. Additionally, in mice with reduced acetylcholine levels in the forebrain via overexpression of A53T α-synuclein (ie, a mouse model of dementia with Lewy bodies and Parkinson's disease with dementia), T-495, like donepezil, reversed the memory deficits in the contextual fear conditioning test and Y-maze task. Thus, low cooperative M1 R PAMs are promising agents for the treatment of memory deficits associated with cholinergic dysfunction.
Keywords: M1 muscarinic acetylcholine receptor; cooperativity; positive allosteric modulator; α-synuclein.
© 2020 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare no other conflict of interest.
Figures


Similar articles
-
TAK-071, a novel M1 positive allosteric modulator with low cooperativity, improves cognitive function in rodents with few cholinergic side effects.Neuropsychopharmacology. 2019 Apr;44(5):950-960. doi: 10.1038/s41386-018-0168-8. Epub 2018 Aug 1. Neuropsychopharmacology. 2019. PMID: 30089885 Free PMC article.
-
An Approach to Discovering Novel Muscarinic M1 Receptor Positive Allosteric Modulators with Potent Cognitive Improvement and Minimized Gastrointestinal Dysfunction.J Pharmacol Exp Ther. 2018 Jan;364(1):28-37. doi: 10.1124/jpet.117.243774. Epub 2017 Oct 12. J Pharmacol Exp Ther. 2018. PMID: 29025977
-
Therapeutic potential of TAK-071, a muscarinic M1 receptor positive allosteric modulator with low cooperativity, for the treatment of cognitive deficits and negative symptoms associated with schizophrenia.Neurosci Lett. 2021 Nov 1;764:136240. doi: 10.1016/j.neulet.2021.136240. Epub 2021 Sep 10. Neurosci Lett. 2021. PMID: 34509568
-
M1 receptor positive allosteric modulators discovery approaches.Trends Pharmacol Sci. 2025 Apr;46(4):298-302. doi: 10.1016/j.tips.2025.03.001. Epub 2025 Mar 25. Trends Pharmacol Sci. 2025. PMID: 40133192 Review.
-
Opportunities and challenges for the development of M1 muscarinic receptor positive allosteric modulators in the treatment for neurocognitive deficits.Br J Pharmacol. 2024 Jul;181(14):2114-2142. doi: 10.1111/bph.15982. Epub 2022 Dec 4. Br J Pharmacol. 2024. PMID: 36355830 Review.
Cited by
-
Clinical and Preclinical Evidence for M1 Muscarinic Acetylcholine Receptor Potentiation as a Therapeutic Approach for Rett Syndrome.Neurotherapeutics. 2022 Jul;19(4):1340-1352. doi: 10.1007/s13311-022-01254-3. Epub 2022 Jun 7. Neurotherapeutics. 2022. PMID: 35670902 Free PMC article.
-
Modulation of arousal and sleep/wake architecture by M1 PAM VU0453595 across young and aged rodents and nonhuman primates.Neuropsychopharmacology. 2020 Dec;45(13):2219-2228. doi: 10.1038/s41386-020-00812-7. Epub 2020 Aug 31. Neuropsychopharmacology. 2020. PMID: 32868847 Free PMC article.
-
Visual hallucinations in Parkinson's disease: spotlight on central cholinergic dysfunction.Brain. 2025 Feb 3;148(2):376-393. doi: 10.1093/brain/awae289. Brain. 2025. PMID: 39252645 Free PMC article. Review.
-
Structure, function and drug discovery of GPCR signaling.Mol Biomed. 2023 Dec 4;4(1):46. doi: 10.1186/s43556-023-00156-w. Mol Biomed. 2023. PMID: 38047990 Free PMC article. Review.
-
Allosteric modulation of G protein-coupled receptor signaling.Front Endocrinol (Lausanne). 2023 Feb 16;14:1137604. doi: 10.3389/fendo.2023.1137604. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36875468 Free PMC article. Review.
References
-
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205‐218. - PubMed
-
- Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev. 1995;21:285‐300. - PubMed
-
- Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology. 2000;54:407‐411. - PubMed
-
- Ballard C, Piggott M, Johnson M, et al. Delusions associated with elevated muscarinic binding in dementia with Lewy bodies. Ann Neurol. 2000;48:868‐876. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

