Advances in Conjugated Microporous Polymers
- PMID: 31990527
- PMCID: PMC7145355
- DOI: 10.1021/acs.chemrev.9b00399
Advances in Conjugated Microporous Polymers
Abstract
Conjugated microporous polymers (CMPs) are a unique class of materials that combine extended π-conjugation with a permanently microporous skeleton. Since their discovery in 2007, CMPs have become established as an important subclass of porous materials. A wide range of synthetic building blocks and network-forming reactions offers an enormous variety of CMPs with different properties and structures. This has allowed CMPs to be developed for gas adsorption and separations, chemical adsorption and encapsulation, heterogeneous catalysis, photoredox catalysis, light emittance, sensing, energy storage, biological applications, and solar fuels production. Here we review the progress of CMP research since its beginnings and offer an outlook for where these materials might be headed in the future. We also compare the prospect for CMPs against the growing range of conjugated crystalline covalent organic frameworks (COFs).
Conflict of interest statement
The authors declare no competing financial interest.
Figures











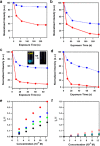


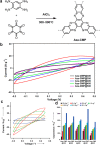




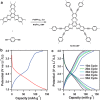
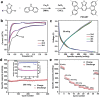













References
-
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051.10.1515/pac-2014-1117. - DOI
-
- Kondo M.; Yoshitomi T.; Matsuzaka H.; Kitagawa S.; Seki K. Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4, 4′-bpy)3(NO3)4]·XH2O}n (M = Co, Ni, Zn). Angew. Chem., Int. Ed. Engl. 1997, 36, 1725–1727. 10.1002/anie.199717251. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

