Metalloproteinase PAPP-A regulation of IGF-1 contributes to polycystic kidney disease pathogenesis
- PMID: 31990681
- PMCID: PMC7101148
- DOI: 10.1172/jci.insight.135700
Metalloproteinase PAPP-A regulation of IGF-1 contributes to polycystic kidney disease pathogenesis
Abstract
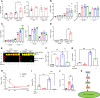
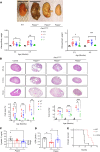
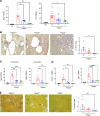
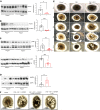
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic cause of end-stage renal disease (ESRD). The treatment options for ADPKD are limited. We observed an upregulation in several IGF-1 pathway genes in the kidney of Pkd1RC/RC mice, a model of ADPKD. Pregnancy-associated plasma protein A (PAPP-A), a metalloproteinase that cleaves inhibitory IGF binding proteins (IGFBPs), increasing the local bioactivity of IGF-1, was highly induced in the kidney of ADPKD mice. PAPP-A levels were high in cystic fluid and kidneys of humans with ADPKD. Our studies further showed that PAPP-A transcription in ADPKD was mainly regulated through the cAMP/CREB/CBP/p300 pathway. Pappa deficiency effectively inhibited the development of cysts in the Pkd1RC/RC mice. The role of PAPP-A in cystic disease appears to be regulation of the IGF-1 pathway and cellular proliferation in the kidney. Finally, preclinical studies demonstrated that treatment with a monoclonal antibody that blocks the proteolytic activity of PAPP-A against IGFBP4 ameliorated ADPKD cystic disease in vivo in Pkd1RC/RC mice and ex vivo in embryonic kidneys. These data indicated that the PAPP-A/IGF-1 pathway plays an important role in the growth and expansion of cysts in ADPKD. Our findings introduce a therapeutic strategy for ADPKD that involves the inhibition of PAPP-A.
Keywords: Molecular pathology; Nephrology.
Conflict of interest statement
Figures


References
-
- Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011;1812(10):1202–1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

