Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network
- PMID: 31991029
- PMCID: PMC8041491
- DOI: 10.1111/liv.14393
Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network
Abstract
Background & aims: Ashwagandha (Withania somnifera) is widely used in Indian Ayurvedic medicine. Several dietary supplements containing ashwagandha are marketed in the US and Europe, but only one case of drug-induced liver injury (DILI) due to ashwagandha has been published. The aim of this case series was to describe the clinical phenotype of suspected ashwagandha-induced liver injury.
Methods: Five cases of liver injury attributed to ashwagandha-containing supplements were identified; three were collected in Iceland during 2017-2018 and two from the Drug-Induced Liver Injury Network (DILIN) in 2016. Other causes for liver injury were excluded. Causality was assessed using the DILIN structured expert opinion causality approach.
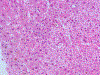
Results: Among the five patients, three were males; mean age was 43 years (range 21-62). All patients developed jaundice and symptoms such as nausea, lethargy, pruritus and abdominal discomfort after a latency of 2-12 weeks. Liver injury was cholestatic or mixed (R ratios 1.4-3.3). Pruritus and hyperbilirubinaemia were prolonged (5-20 weeks). No patient developed hepatic failure. Liver tests normalized within 1-5 months in four patients. One patient was lost to follow-up. One biopsy was performed, showing acute cholestatic hepatitis. Chemical analysis confirmed ashwagandha in available supplements; no other toxic compounds were identified. No patient was taking potentially hepatotoxic prescription medications, although four were consuming additional supplements, and in one case, rhodiola was a possible causative agent along with ashwagandha.
Conclusions: These cases illustrate the hepatotoxic potential of ashwagandha. Liver injury is typically cholestatic or mixed with severe jaundice and pruritus, but self-limited with liver tests normalizing in 1-5 months.
Keywords: dietary supplements; drug-induced liver injury; liver.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures
Comment in
-
An Eye into the Allegations about Ashwagandha.Liver Int. 2020 Aug;40(8):2034-2035. doi: 10.1111/liv.14459. Epub 2020 Apr 21. Liver Int. 2020. PMID: 32267603 No abstract available.
-
Ashwagandha as a cause for liver injury.Liver Int. 2020 Aug;40(8):2035-2036. doi: 10.1111/liv.14551. Epub 2020 Jun 11. Liver Int. 2020. PMID: 32475004 No abstract available.
References
-
- Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–1425, 1425.e1411–1413; quiz e1419–1420. - PubMed
-
- Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical