Cobalt-Catalyzed Hydrogen-Atom Transfer Induces Bicyclizations that Tolerate Electron-Rich and Electron-Deficient Intermediate Alkenes
- PMID: 31991035
- PMCID: PMC7124983
- DOI: 10.1002/anie.202000252
Cobalt-Catalyzed Hydrogen-Atom Transfer Induces Bicyclizations that Tolerate Electron-Rich and Electron-Deficient Intermediate Alkenes
Abstract
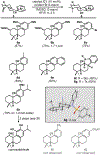
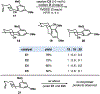
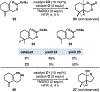
A novel CoII -catalyzed polyene cyclization was developed that is uniquely effective when performed in hexafluoroisopropanol as the solvent. The process is presumably initiated by metal-catalyzed hydrogen-atom transfer (MHAT) to 1,1-disubstituted or monosubstituted alkenes, and the reaction is remarkable for its tolerance of internal alkenes bearing either electron-rich methyl or electron-deficient nitrile substituents. Electron-rich aromatic terminators are required in both cases. Terpenoid scaffolds with different substitution patterns are obtained with excellent diastereoselectivities, and the bioactive C20-oxidized abietane diterpenoid carnosaldehyde was made to showcase the utility of the nitrile-bearing products. Also provided are the results of several mechanistic experiments that suggest the process features an MHAT-induced radical bicyclization with late-stage oxidation to regenerate the aromatic terminator.
Keywords: cobalt; cyclizations; polycycles; reaction mechanisms; terpenoids.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
-
- For representative pioneering achievements, see:
- Stork G, Burgstahler AW, J. Am. Chem. Soc 1955, 77, 5068–5077;
- Eschenmoser A, Ruzicka L, Jeger O, Arigoni D, Helv. Chim. Acta 1955, 38, 1890–1904;
- Johnson WS, Acc. Chem. Res 1968, 1, 1–8;
- van Tamelen EE, Willet J, Schwartz M, Nadeau R, J. Am. Chem. Soc 1966, 88, 5937–5938. - PubMed
-
- For selected examples of radical polycyclizations that mirror the biomimetic cationic reactions of the type described in this disclosure, see:
- Rendler S and MacMillan DWC, J. Am. Chem. Soc 2010, 132, 5027–5029; - PMC - PubMed
- Barrero AF, Cuerva JM, Herrador MM, Valdivia MV, J. Org. Chem 2001, 66, 4074–4078; - PubMed
- Chen L, Gill GB, Pattenden G, Simonian H, J. Chem. Soc., Perkin Trans 1, 1996, 31–43;
- Zoretic PA, Fang H, Ribeiro AA, J. Org. Chem 1998, 63, 7213–7217; - PubMed
- Snider BB, Mohan RM, Kates SA, J. Org. Chem 1985, 50, 3659;
- Breslow R, Olin SS, Groves JT, Tetrahedron Lett. 1968, 9, 1837–1840.
-
- For selected examples of organometallic polycyclizations that mirror the biomimetic cationic reactions of the type described in this disclosure, see:
- Mullen CA, Gagné MR, J. Am. Chem. Soc 2007, 129, 11880–11881. - PubMed
- Schafroth MA, Sarlah D, Krautwald S, Carreira EM, J. Am. Chem. Soc 2012, 134, 20276–20278. - PubMed
-
- Wang FP, Chen QH, Liu XY, Nat. Prod. Rep 1999, 16, 529–570. - PubMed

