The biochemistry of headgroup exchange during triacylglycerol synthesis in canola
- PMID: 31991038
- PMCID: PMC7605783
- DOI: 10.1111/tpj.14709
The biochemistry of headgroup exchange during triacylglycerol synthesis in canola
Abstract
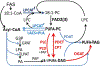
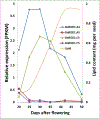
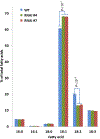
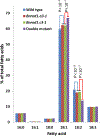
Many pathways of primary metabolism are substantially conserved within and across plant families. However, significant differences in organization and fluxes through a reaction network may occur, even between plants in closely related genera. Assessing and understanding these differences is key to appreciating metabolic diversity, and to attempts to engineer plant metabolism for higher crop yields and desired product profiles. To better understand lipid metabolism and seed oil synthesis in canola (Brassica napus), we have characterized four canola homologues of the Arabidopsis (Arabidopsis thaliana) ROD1 gene. AtROD1 encodes phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT), the enzyme that catalyzes a major flux of polyunsaturated fatty acids (PUFAs) in oil synthesis. Assays in yeast indicated that only two of the canola genes, BnROD1.A3 and BnROD1.C3, encode active isozymes of PDCT, and these genes are strongly expressed during the period of seed oil synthesis. Loss of expression of BnROD1.A3 and BnROD1.C3 in a double mutant, or by RNA interference, reduced the PUFA content of the oil to 26.6% compared with 32.5% in the wild type. These results indicate that ROD1 isozymes in canola are responsible for less than 20% of the PUFAs that accumulate in the seed oil compared with 40% in Arabidopsis. Our results demonstrate the care needed when translating results from a model species to crop plants.
Keywords: ROD1; fatty acids; metabolic diversity; seed lipid metabolism; seed oil; triacylglycerol.
© 2020 The Authors The Plant Journal © 2020 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare no competing financial interests.
Figures




References
-
- Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ (1990) Basic local alignment search tool. J. Mol. Biol 215, 403–410. - PubMed
-
- Bates PD, Ohlrogge JB and Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem 282, 31206–31216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

