Targeting SOX2 Protein with Peptide Aptamers for Therapeutic Gains against Esophageal Squamous Cell Carcinoma
- PMID: 31991109
- PMCID: PMC7054732
- DOI: 10.1016/j.ymthe.2020.01.012
Targeting SOX2 Protein with Peptide Aptamers for Therapeutic Gains against Esophageal Squamous Cell Carcinoma
Abstract
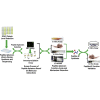
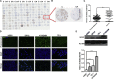
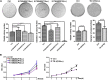
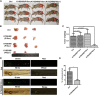
Esophageal squamous cell carcinoma (ESCC) is a predominant cancer type in developing countries such as China, where ESCC accounts for approximately 90% of esophageal malignancies. Lacking effective and targeted therapy contributes to the poor 5-year survival rate. Recent studies showed that about 30% of ESCC cases have high levels of SOX2. Herein, we aim to target this transcription factor with aptamer. We established a peptide aptamer library and then performed an unbiased screening to identify several peptide aptamers including P42 that can bind and inhibit SOX2 downstream target genes. We further found that P42 overexpression or incubation with a synthetic peptide 42 inhibited the proliferation, migration, and invasion of ESCC cells. Moreover, peptide 42 treatment inhibited the growth and metastasis of ESCC xenografts in mouse and zebrafish. Further analysis revealed that P42 overexpression led to alternations in the levels of proteins that are important for the proliferation and migration of ESCC cells. Taken together, our study identified the peptide 42 as a key inhibitor of SOX2 function, reducing the proliferation and migration of ESCC cells in vitro and in vivo, and thereby offering a potential therapy against ESCC.
Keywords: SOX2; drug screen; esophageal squamous cell carcinoma; peptide; peptide aptamers.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Prabhu A., Obi K.O., Rubenstein J.H. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am. J. Gastroenterol. 2014;109:822–827. - PubMed
-
- Chang F., Syrjänen S., Wang L., Syrjänen K. Infectious agents in the etiology of esophageal cancer. Gastroenterology. 1992;103:1336–1348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

