Cardiac Nestin+ Mesenchymal Stromal Cells Enhance Healing of Ischemic Heart through Periostin-Mediated M2 Macrophage Polarization
- PMID: 31991111
- PMCID: PMC7054724
- DOI: 10.1016/j.ymthe.2020.01.011
Cardiac Nestin+ Mesenchymal Stromal Cells Enhance Healing of Ischemic Heart through Periostin-Mediated M2 Macrophage Polarization
Abstract
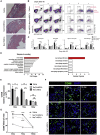
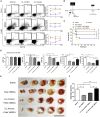
Mesenchymal stromal cells (MSCs) show potential for treating cardiovascular diseases, but their therapeutic efficacy exhibits significant heterogeneity depending on the tissue of origin. This study sought to identify an optimal source of MSCs for cardiovascular disease therapy. We demonstrated that Nestin was a suitable marker for cardiac MSCs (Nes+cMSCs), which were identified by their self-renewal ability, tri-lineage differentiation potential, and expression of MSC markers. Furthermore, compared with bone marrow-derived MSCs (Nes+bmMSCs) or saline-treated myocardial infarction (MI) controls, intramyocardial injection of Nes+cMSCs significantly improved cardiac function and decreased infarct size after acute MI (AMI) through paracrine actions, rather than transdifferentiation into cardiac cells in infarcted heart. We further revealed that Nes+cMSC treatment notably reduced pan-macrophage infiltration while inducing macrophages toward an anti-inflammatory M2 phenotype in ischemic myocardium. Interestingly, Periostin, which was highly expressed in Nes+cMSCs, could promote the polarization of M2-subtype macrophages, and knockdown or neutralization of Periostin remarkably reduced the therapeutic effects of Nes+cMSCs by decreasing M2 macrophages at lesion sites. Thus, the present work systemically shows that Nes+cMSCs have greater efficacy than do Nes+bmMSCs for cardiac healing after AMI, and that this occurs at least partly through Periostin-mediated M2 macrophage polarization.
Keywords: Periostin; immunomodulation; macrophage polarization; mesenchymal stromal cells; myocardial infarction.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway.BMC Med. 2023 Mar 16;21(1):96. doi: 10.1186/s12916-023-02778-x. BMC Med. 2023. PMID: 36927608 Free PMC article.
-
Bone-derived Nestin-positive mesenchymal stem cells improve cardiac function via recruiting cardiac endothelial cells after myocardial infarction.Stem Cell Res Ther. 2019 Apr 27;10(1):127. doi: 10.1186/s13287-019-1217-x. Stem Cell Res Ther. 2019. PMID: 31029167 Free PMC article.
-
Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages.Exp Mol Med. 2014 Jan 10;46(1):e70. doi: 10.1038/emm.2013.135. Exp Mol Med. 2014. PMID: 24406319 Free PMC article.
-
Extracardiac-Lodged Mesenchymal Stromal Cells Propel an Inflammatory Response Against Myocardial Infarction via Paracrine Effects.Cell Transplant. 2016;25(5):929-35. doi: 10.3727/096368915X689758. Epub 2015 Oct 22. Cell Transplant. 2016. PMID: 26498018 Review.
-
The Role of Mesenchymal Stem/Stromal Cells Secretome in Macrophage Polarization: Perspectives on Treating Inflammatory Diseases.Curr Stem Cell Res Ther. 2024;19(6):894-905. doi: 10.2174/1574888X18666230811093101. Curr Stem Cell Res Ther. 2024. PMID: 37723965 Review.
Cited by
-
Cardiac Derived CD51-Positive Mesenchymal Stem Cells Enhance the Cardiac Repair Through SCF-Mediated Angiogenesis in Mice With Myocardial Infarction.Front Cell Dev Biol. 2021 Apr 21;9:642533. doi: 10.3389/fcell.2021.642533. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968928 Free PMC article.
-
Effects of Lipids and Lipoproteins on Mesenchymal Stem Cells Used in Cardiac Tissue Regeneration.Int J Mol Sci. 2020 Jul 5;21(13):4770. doi: 10.3390/ijms21134770. Int J Mol Sci. 2020. PMID: 32635662 Free PMC article. Review.
-
Umbilical cord-derived mesenchymal stem cells preferentially modulate macrophages to alleviate pulmonary fibrosis.Stem Cell Res Ther. 2024 Dec 18;15(1):475. doi: 10.1186/s13287-024-04091-7. Stem Cell Res Ther. 2024. PMID: 39696548 Free PMC article.
-
Periostin+ myeloid cells improved long bone regeneration in a mechanosensitive manner.Bone Res. 2024 Oct 15;12(1):59. doi: 10.1038/s41413-024-00361-5. Bone Res. 2024. PMID: 39406726 Free PMC article.
-
Dental Follicle Stem Cells Promote Periodontal Regeneration through Periostin-Mediated Macrophage Infiltration and Reprogramming in an Inflammatory Microenvironment.Int J Mol Sci. 2023 Mar 28;24(7):6353. doi: 10.3390/ijms24076353. Int J Mol Sci. 2023. PMID: 37047322 Free PMC article.
References
-
- Lloyd-Jones D., Adams R.J., Brown T.M., Carnethon M., Dai S., De Simone G., Ferguson T.B., Ford E., Furie K., Gillespie C., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. - PubMed
-
- Okwuosa I.S., Lewsey S.C., Adesiyun T., Blumenthal R.S., Yancy C.W. Worldwide disparities in cardiovascular disease: challenges and solutions. Int. J. Cardiol. 2016;202:433–440. - PubMed
-
- Oh H., Ito H., Sano S. Challenges to success in heart failure: cardiac cell therapies in patients with heart diseases. J. Cardiol. 2016;68:361–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

