Chapter 22: Structural and signaling functions of integrins
- PMID: 31991120
- PMCID: PMC7063833
- DOI: 10.1016/j.bbamem.2020.183206
Chapter 22: Structural and signaling functions of integrins
Abstract
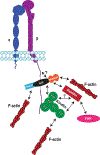
The integrin family of transmembrane adhesion receptors is essential for sensing and adhering to the extracellular environment. Integrins are heterodimers composed of non-covalently associated α and β subunits that engage extracellular matrix proteins and couple to intracellular signaling and cytoskeletal complexes. Humans have 24 different integrin heterodimers with differing ligand binding specificities and non-redundant functions. Complex structural rearrangements control the ability of integrins to engage ligands and to activate diverse downstream signaling networks, modulating cell adhesion and dynamics, processes which are crucial for metazoan life and development. Here we review the structural and signaling functions of integrins focusing on recent advances which have enhanced our understanding of how integrins are activated and regulated, and the cytoplasmic signaling networks downstream of integrins.
Keywords: Cytoskeleton; Focal adhesion; Integrin; Kindlin; Talin.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


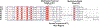
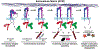
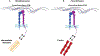
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

