The role of hepcidin and iron homeostasis in atherosclerosis
- PMID: 31991168
- PMCID: PMC7066581
- DOI: 10.1016/j.phrs.2020.104664
The role of hepcidin and iron homeostasis in atherosclerosis
Abstract
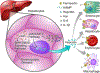
Atherosclerotic cardiovascular disease is a major burden on global health and a leading cause of death worldwide. The pathophysiology of this chronic disease is complex, involving inflammation, lipoprotein oxidation and accumulation, plaque formation, and calcification. In 1981, Dr. Jerome Sullivan formulated the 'Iron Hypothesis', suggesting that higher levels of stored iron promote cardiovascular diseases, whereas iron deficiency may have an atheroprotective effect. This hypothesis has stimulated research focused on clarifying the role of iron in the development of atherosclerosis. However, preclinical and clinical studies have produced contradictory results and the observation that patients with hemochromatosis do not appear to have an increased risk of atherosclerosis seemed incongruous with Sullivan's initial hypothesis. The 'paradox' of systemic iron overload not being accompanied by an increased risk for atherosclerosis led to a refinement of the iron hypothesis focusing on intracellular macrophage iron. More recent in vitro and animal studies have elucidated the complex signaling pathways regulating iron, with a particular focus on hepcidin, the master regulator of body iron homeostasis. Bone morphogenetic protein (BMP) signaling is the major pathway that is required for induction of hepcidin expression in response to increasing levels of iron. Strong links between iron homeostasis, BMP signaling, inflammation and atherosclerosis have been established in both mechanistic and human studies. This review summarizes the current understanding of the role of iron homeostasis and hepcidin in the development of atherosclerosis and discusses the BMP-hepcidin-ferroportin axis as a novel therapeutic target for the treatment of cardiovascular disease.
Keywords: Atherosclerosis; Bone morphogenetic proteins; Hepcidin; Inflammation; Iron; Macrophages.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by bindirg to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. - PubMed
-
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. - PubMed
-
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl), C7–12. - PubMed
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating. the biology of atherosclerosis. Nature. 2011;473(7347):317–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

