FMRP regulates presynaptic localization of neuronal voltage gated calcium channels
- PMID: 31991246
- PMCID: PMC7152798
- DOI: 10.1016/j.nbd.2020.104779
FMRP regulates presynaptic localization of neuronal voltage gated calcium channels
Abstract
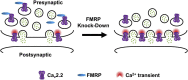
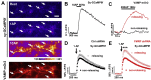
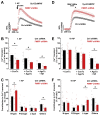
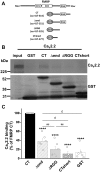
Fragile X syndrome (FXS), the most common form of inherited intellectual disability and autism, results from the loss of fragile X mental retardation protein (FMRP). We have recently identified a direct interaction of FMRP with voltage-gated Ca2+ channels that modulates neurotransmitter release. In the present study we used a combination of optophysiological tools to investigate the impact of FMRP on the targeting of voltage-gated Ca2+ channels to the active zones in neuronal presynaptic terminals. We monitored Ca2+ transients at synaptic boutons of dorsal root ganglion (DRG) neurons using the genetically-encoded Ca2+ indicator GCaMP6f tagged to synaptophysin. We show that knock-down of FMRP induces an increase of the amplitude of the Ca2+ transient in functionally-releasing presynaptic terminals, and that this effect is due to an increase of N-type Ca2+ channel contribution to the total Ca2+ transient. Dynamic regulation of CaV2.2 channel trafficking is key to the function of these channels in neurons. Using a CaV2.2 construct with an α-bungarotoxin binding site tag, we further investigate the impact of FMRP on the trafficking of CaV2.2 channels. We show that forward trafficking of CaV2.2 channels from the endoplasmic reticulum to the plasma membrane is reduced when co-expressed with FMRP. Altogether our data reveal a critical role of FMRP on localization of CaV channels to the presynaptic terminals and how its defect in a context of FXS can profoundly affect synaptic transmission.
Keywords: Calcium transients; Fragile X syndrome - FMRP; Synaptic transmission; Trafficking; Voltage-gated calcium channels.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures


References
-
- Abriel H., Staub O. Ubiquitylation of ion channels. Physiology (Bethesda) 2005;20:398–407. - PubMed
-
- Albuquerque C., Joseph D.J., Choudhury P., MacDermott A.B. Cold Spring Harb Protoc 2009, pdb prot5275. 2009. Dissection, plating, and maintenance of dorsal root ganglion neurons for monoculture and for coculture with dorsal horn neurons. - PubMed
-
- Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H.W., Hermosilla T., Zamponi G.W. The Cavbeta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat. Neurosci. 2011;14:173–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

