Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells
- PMID: 31991567
- PMCID: PMC7071193
- DOI: 10.3390/nu12020309
Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells
Abstract
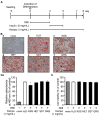
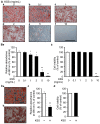
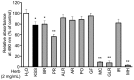
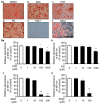
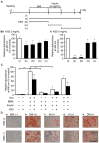
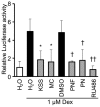
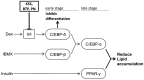
Excessive triglyceride accumulation in lipid-metabolizing tissues is associated with an increased risk of a variety of metabolic diseases. Kamishoyosan (KSS) is a Kampo composed of 10 constituent herbs, and contains moutan cortex (MC) and paeonol (PN) as the major ingredient of MC. Here, we demonstrate the molecular mechanism underlying the effect of KSS on the differentiation of mouse preadipocytes (3T3-L1 cells). KSS inhibited the accumulation of triglycerides in a dose-dependent manner in 3T3-L1 cells that were induced to differentiate into adipocytes. We also found that MC and PN were responsible for the anti-adipogenetic effect of KSS and significantly suppressed the expression of CCAAT/enhancer-binding proteins-δ (C/EBP-δ) mRNA 3 days after the induction of differentiation. Thus, PN may contribute to the anti-adipogenetic property of MC in 3T3-L1 cells. In addition, PN inhibited dexamethasone (Dex)-induced glucocorticoid receptor (GR) promoter activity. Taken together, these results suggest that PN suppresses C/EBP-δ expression by inhibiting Dex-induced GR promoter activity at the early stage of differentiation and, consequently, delays differentiation into mature adipocytes. Our results suggest that the habitual intake of Kampo-containing PN contributes to the prevention of the onset of metabolic diseases by decreasing the excessive accumulation of triglycerides in lipid-metabolizing tissues.
Keywords: C/EBP-δ; Kamishoyosan; Kampo medicine; Moutan cortex; adipocyte; glucocorticoid receptor; paeonol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
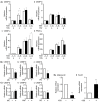
References
-
- Kivipelto M., Ngandu T., Fratiglioni L., Viitanen M., Kareholt I., Winblad B., Helkala E.L., Tuomilehto J., Soininen H., Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

