Solubility Enhancement of Myricetin by Inclusion Complexation with Heptakis- O-(2-Hydroxypropyl)-β-Cyclodextrin: A Joint Experimental and Theoretical Study
- PMID: 31991574
- PMCID: PMC7038215
- DOI: 10.3390/ijms21030766
Solubility Enhancement of Myricetin by Inclusion Complexation with Heptakis- O-(2-Hydroxypropyl)-β-Cyclodextrin: A Joint Experimental and Theoretical Study
Abstract
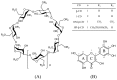
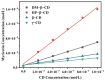
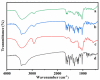
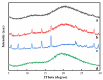
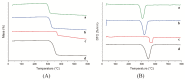
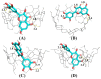
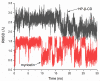
Four cyclodextrins (CD) including β-cyclodextrin (β-CD), γ-cyclodextrin (γ-CD), heptakis-O-(2-hydroxypropyl)-β-cyclodextrin (HP-β-CD), and heptakis-O-(2, 6-di-O-methyl)-β-cyclodextrin (DM-β-CD) were used as solubilizer to study the solubility enhancement of myricetin. The results of the phase solubility study showed that the presence of CDs could enhance the solubility of myricetin by forming 1:1 complexes. Among all CDs, HP-β-CD had the highest solubilization effect to myricetin. The concentration of myricetin could be 1.60 × 10-4 moL/L when the presence of HP-β-CD reached 1.00 × 10-2 moL/L, which was 31.45 times higher than myricetin's aqueous solubility. Subsequently, the HP-β-CD:myricetin complex was characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). In order to get an insight of the plausible structure of the complex, molecular docking was used to study the complexation process of HP-β-CD and myricetin. In the complex, the A ring and C ring of myricetin were complexed into the hydrophobic cavity of HP-β-CD, while the ring B was located at the wide rim of HP-β-CD. Four hydrogen bonding interactions were found between HP-β-CD and -OH groups of the guest in the HP-β-CD: myricetin complex. The complexation energy (△E) for the host-guest interactions was calculated with a negative sign, indicating the formation of the complex was an exergonic process. A 30-ns molecular dynamics simulation was conducted to the HP-β-CD: myricetin complex. Calculation results showed that no large structural deformation or position change were observed during the whole simulation time span. The average root-mean-square deviation (RMSD) changes of the host and guest were 2.444 and 1.145 Å, respectively, indicating the complex had excellent stability.
Keywords: HP-β-CD; Keywords myricetin; PM6-D3H4; PM7; molecular dynamics; phase solubility.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. 1980;19:344–362. doi: 10.1002/anie.198003441. - DOI
-
- Singh R., Bharti N., Madan J., Hiremath S. Characterization of cyclodextrin inclusion complexes—A review. J. Pharm. Sci. Technol. 2010;2:171–183.
-
- Swaminathan S., Pastero L., Serpe L., Trotta F., Vavia P., Aquilano D., Trotta M., Zara G., Cavalli R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010;74:193–201. doi: 10.1016/j.ejpb.2009.11.003. - DOI - PubMed
-
- Wang J., Cao Y., Sun B., Wang C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011;127:1680–1685. doi: 10.1016/j.foodchem.2011.02.036. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

