Advanced Methodology and Preliminary Measurements of Molecular and Mechanical Properties of Heart Valves under Dynamic Strain
- PMID: 31991583
- PMCID: PMC7037596
- DOI: 10.3390/ijms21030763
Advanced Methodology and Preliminary Measurements of Molecular and Mechanical Properties of Heart Valves under Dynamic Strain
Abstract
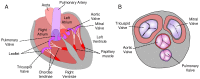
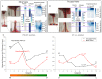
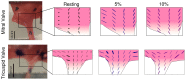
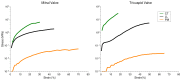
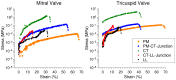
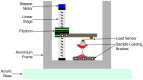
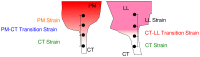
Mammalian heart valves are soft tissue assemblies with multi-scale material properties. This is because they are constructs comprising both muscle and non-contractile extracellular matrix proteins (such as collagens and proteoglycans) and transition regions where one form of tissue structure becomes another, significantly different form. The leaflets of the mitral and tricuspid valves are connected to chordae tendinae which, in turn, bind through papillary muscles to the cardiac wall of the ventricle. The transition regions between these tissue subsets are complex and diffuse. Their material composition and mechanical properties have not been previously described with both micro and nanoscopic data recorded simultaneously, as reported here. Annotating the mechanical characteristics of these tissue transitions will be of great value in developing novel implants, improving the state of the surgical simulators and advancing robot-assisted surgery. We present here developments in multi-scale methodology that produce data that can relate mechanical properties to molecular structure using scanning X-ray diffraction. We correlate these data to corresponding tissue level (macro and microscopic) stress and strain, with particular emphasis on the transition regions and present analyses to indicate points of possible failure in these tissues.
Keywords: X-ray diffraction scanning; heart valves; stress–strain relations; tissue transition regions; valve failure.
Conflict of interest statement
The authors disclose a perceived conflict of interest for this edition in that the senior author (J.P.R.O.O) is an editor of the “Molecular Tissue Responses to Mechanical Loading” special edition and a member of the editorial board. The co-editor, Ashley Eidsmore, was also a co-author of a manuscript with the lead and senior authors within the last 12 months. The authors declare no such relationship or affiliation with any of the suggested reviewers, nor any other known conflicts or perceived conflicts.
Figures

References
-
- Vukanovic-Criley J.M., Criley S., Warde C.M., Boker J.R., Guevara-Matheus L., Churchill W.H., Nelson W.P., Criley J.M. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: A multicenter study. Arch. Intern. Med. 2006;166:610–616. doi: 10.1001/archinte.166.6.610. - DOI - PubMed
-
- Stephens E.H., Chu C.K., Grande-Allen K.J. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008;4:1148–1160. doi: 10.1016/j.actbio.2008.03.014. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

