Endothelial response boosted by platelet lysate: the involvement of calcium toolkit
- PMID: 31991927
- PMCID: PMC7036775
- DOI: 10.3390/ijms21030808
Endothelial response boosted by platelet lysate: the involvement of calcium toolkit
Abstract
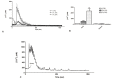
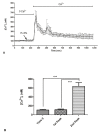
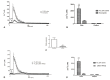
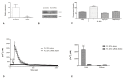
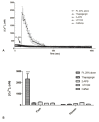
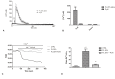
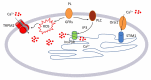
Wound repair is a dynamic process during which crucial signaling pathways are regulated by growth factors and cytokines released by several kinds of cells directly involved in the healing process. However, the limited applications and heterogeneous clinical results of single growth factors in wound healing encouraged the use of a mixture of bioactive molecules such as platelet derivatives for best results in wound repair. An interesting platelet derivative, obtained from blood samples, is platelet lysate (PL), which has shown potential clinical application. PL is obtained from freezing and thawing of platelet-enriched blood samples. Intracellular calcium (Ca2+) signals play a central role in the control of endothelial cell survival, proliferation, motility, and differentiation. We investigated the role of Ca2+ signaling in the PL-driven endothelial healing process. In our experiments, the functional significance of Ca2+ signaling machinery was highlighted performing the scratch wound assay in presence of different inhibitors or specific RNAi. We also pointed out that the PL-induced generation of intracellular ROS (reactive oxygen species) via NOX4 (NADPH oxidase 4) is necessary for the activation of TRPM2 and the resulting Ca2+ entry from the extracellular space. This is the first report of the mechanism of wound repair in an endothelial cell model boosted by the PL-induced regulation of [Ca2+]i.
Keywords: ROS; cell calcium; endothelial cells; platelet lysate; wound repair.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures


References
-
- Ranzato E., Burlando B. Signaling pathways in wound repair. In: Middleton J.E., editor. Wound Healing: Process, Phases and Promoting. Nova Publishers Inc.; Hauppauge, NY, USA: 2011.
-
- Martinotti S., Ranzato E. Dynamic interplay between cell types during wound healing. In: Ranzato E., editor. Keratinocytes: Structure, Molecular Mechanisms and Role in Immunity. Nova Publishers Inc.; Hauppauge, NY, USA: 2013.
-
- Ranzato E., Mazzucco L., Burlando B. Platelet Derivatives: A New Horizon in Regenerative Medicine. In: Lv B., editor. Advances in Medicine and Biology. Nova Publishers Inc.; Hauppauge, NY, USA: 2010.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

