Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel
- PMID: 31992046
- PMCID: PMC7218745
- DOI: 10.1021/acs.biomac.9b01745
Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel
Abstract
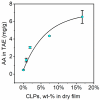
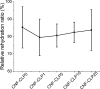
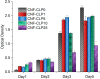
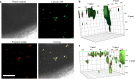
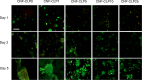
Three-dimensional (3D) printing has been an emerging technique to fabricate precise scaffolds for biomedical applications. Cellulose nanofibril (CNF) hydrogels have attracted considerable attention as a material for 3D printing because of their shear-thinning properties. Combining cellulose nanofibril hydrogels with alginate is an effective method to enable cross-linking of the printed scaffolds in the presence of Ca2+ ions. In this work, spherical colloidal lignin particles (CLPs, also known as spherical lignin nanoparticles) were used to prepare CNF-alginate-CLP nanocomposite scaffolds. High-resolution images obtained by atomic force microscopy (AFM) showed that CLPs were homogeneously mixed with the CNF hydrogel. CLPs brought antioxidant properties to the CNF-alginate-CLP scaffolds in a concentration-dependent manner and increased the viscosity of the hydrogels at a low shear rate, which correspondingly provide better shape fidelity and printing resolution to the scaffolds. Interestingly, the CLPs did not affect the viscosity at high shear rates, showing that the shear thinning behavior typical for CNF hydrogels was retained, enabling easy printing. The CNF-alginate-CLP scaffolds demonstrated shape stability after printing, cross-linking, and storage in Dulbecco's phosphate buffer solution (DPBS +) containing Ca2+ and Mg2+ ions, up to 7 days. The 3D-printed scaffolds showed relative rehydration ratio values above 80% after freeze-drying, demonstrating a high water-retaining capability. Cell viability tests using hepatocellular carcinoma cell line HepG2 showed no negative effect of CLPs on cell proliferation. Fluorescence microscopy indicated that HepG2 cells grew not only on the surfaces but also inside the porous scaffolds. Overall, our results demonstrate that nanocomposite CNF-alginate-CLP scaffolds have high potential in soft-tissue engineering and regenerative-medicine applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
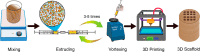



References
-
- Chun H. J.; Park K.; Kim C.-H.; Khang G.. Novel Biomaterials for Regenerative Medicine; Springer, Singapore: Singapore, 2018.
-
- De France K. J.; Badv M.; Dorogin J.; Siebers E.; Panchal V.; Babi M.; Moran-Mirabal J.; Lawlor M.; Cranston E. D.; Hoare T. Tissue Response and Biodistribution of Injectable Cellulose Nanocrystal Composite Hydrogels. ACS Biomater. Sci. Eng. 2019, 5 (5), 2235–2246. 10.1021/acsbiomaterials.9b00522. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

