Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction
- PMID: 31992066
- PMCID: PMC7135928
- DOI: 10.1161/CIRCULATIONAHA.119.041882
Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction
Abstract
Background: Sphingolipids have recently emerged as a biomarker of recurrence and mortality after myocardial infarction (MI). The increased ceramide levels in mammalian heart tissues during acute MI, as demonstrated by several groups, is associated with higher cell death rates in the left ventricle and deteriorated cardiac function. Ceramidase, the only enzyme known to hydrolyze proapoptotic ceramide, generates sphingosine, which is then phosphorylated by sphingosine kinase to produce the prosurvival molecule sphingosine-1-phosphate. We hypothesized that Acid Ceramidase (AC) overexpression would counteract the negative effects of elevated ceramide and promote cell survival, thereby providing cardioprotection after MI.
Methods: We performed transcriptomic, sphingolipid, and protein analyses to evaluate sphingolipid metabolism and signaling post-MI. We investigated the effect of altering ceramide metabolism through a loss (chemical inhibitors) or gain (modified mRNA [modRNA]) of AC function post hypoxia or MI.
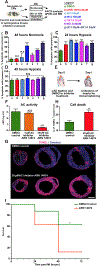
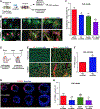
Results: We found that several genes involved in de novo ceramide synthesis were upregulated and that ceramide (C16, C20, C20:1, and C24) levels had significantly increased 24 hours after MI. AC inhibition after hypoxia or MI resulted in reduced AC activity and increased cell death. By contrast, enhancing AC activity via AC modRNA treatment increased cell survival after hypoxia or MI. AC modRNA-treated mice had significantly better heart function, longer survival, and smaller scar size than control mice 28 days post-MI. We attributed the improvement in heart function post-MI after AC modRNA delivery to decreased ceramide levels, lower cell death rates, and changes in the composition of the immune cell population in the left ventricle manifested by lowered abundance of proinflammatory detrimental neutrophils.
Conclusions: Our findings suggest that transiently altering sphingolipid metabolism through AC overexpression is sufficient and necessary to induce cardioprotection post-MI, thereby highlighting the therapeutic potential of AC modRNA in ischemic heart disease.
Keywords: acid ceramidase; cardioprotective agents; mRNA; myocardial infarction; sphingolipids.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
E.E., L.Z, A.S.V. and Y.H. are Inventors on Provisional Patent Application (MODRNA ENCODING SPHINGOLIPID METABOLIZING PROTEINS TO PROMOTE CELL SURVIVAL) 3710/039P, Filed March 2018, which covers the results in this manuscript.
Figures



References
-
- Spencer FA, Meyer TE, Gore JM and Goldberg RJ. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of Myocardial Infarction. Circulation. 2002;105:2605–2610. - PubMed
-
- Meeusen JW, Donato LJ and Jaffe AS. Lipid Biomarkers for Risk Assessment in Acute Coronary Syndromes. Curr Cardiol Rep. 2017;19:48. - PubMed
-
- Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, Bai Y, Schuchman EH, He X and Zhang G. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol. 2015;31:357–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

