Pelvic floor rehabilitation to improve functional outcome and quality of life after surgery for rectal cancer: study protocol for a randomized controlled trial (FORCE trial)
- PMID: 31992358
- PMCID: PMC6988240
- DOI: 10.1186/s13063-019-4043-7
Pelvic floor rehabilitation to improve functional outcome and quality of life after surgery for rectal cancer: study protocol for a randomized controlled trial (FORCE trial)
Abstract
Background: After low anterior resection (LAR), up to 90% of patients develop anorectal dysfunction. Especially fecal incontinence has a major impact on the physical, psychological, social, and emotional functioning of the patient but also on the Dutch National Healthcare budget with more than €2000 spent per patient per year. No standardized treatment is available to help these patients. Common treatment nowadays is focused on symptom relief, consisting of lifestyle advices and pharmacotherapy with bulking agents or antidiarrheal medication. Another possibility is pelvic floor rehabilitation (PFR), which is one of the most important treatments for fecal incontinence in general, with success rates of 50-80%. No strong evidence is available for the use of PFR after LAR. This study aims to prove a beneficial effect of PFR on fecal incontinence, quality of life, and costs in rectal cancer patients after sphincter-saving surgery compared to standard treatment.
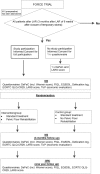
Methods: The FORCE trial is a multicenter, two-armed, randomized clinical trial. All patients that underwent LAR are recruited from the participating hospitals and randomized for either standard treatment or a standardized PFR program. A total of 128 patients should be randomized. Optimal blinding is not possible. Stratification will be done in variable blocks (gender and additional radiotherapy). The primary endpoint is the Wexner incontinence score; secondary endpoints are health-related and fecal-incontinence-related QoL and cost-effectiveness. Baseline measurements take place before randomization. The primary endpoint is measured 3 months after the start of the intervention, with a 1-year follow-up for sustainability research purposes.
Discussion: The results of this study may substantially improve postoperative care for patients with fecal incontinence or anorectal dysfunction after LAR. This section provides insight in the decisions that were made in the organization of this trial.
Trial registration: Netherlands Trial Registration, NTR5469, registered on 03-09-2015. Protocol FORCE trial V18, 19-09-2019. Sponsor Radboud University Medical Center, Nijmegen.
Keywords: Quality of life, Fecal incontinence, Low anterior resection, Pelvic floor rehabilitation, Functional outcomes, Low anterior resection syndrome, Rectal cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous