Dirofilaria immitis possesses molecules with anticoagulant properties in its excretory/secretory antigens
- PMID: 31992384
- PMCID: PMC10317642
- DOI: 10.1017/S0031182020000104
Dirofilaria immitis possesses molecules with anticoagulant properties in its excretory/secretory antigens
Abstract
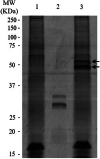
Dirofilaria immitis is a parasitic nematode that survives in the circulatory system of suitable hosts for many years, causing the most severe thromboembolisms when simultaneous death of adult worms occurs. The two main mechanisms responsible for thrombus formation in mammals are the activation and aggregation of platelets and the generation of fibrin through the coagulation cascade. The aim of this work was to study the anticoagulant potential of excretory/secretory antigens from D. immitis adult worms (DiES) on the coagulation cascade of the host. Anticoagulant and inhibition assays respectively showed that DiES partially alter the coagulation cascade of the host and reduce the activity of the coagulation factor Xa, a key enzyme in the coagulation process. In addition, a D. immitis protein was identified by its similarity to the homologous serpin 6 from Brugia malayi as a possible candidate to form an inhibitory complex with FXa by sodium dodecyl sulfate polyacrylamide gel electrophoresis and mass spectrometry. These results indicate that D. immitis could use the anticoagulant properties of its excretory/secretory antigens to control the formation of blood clots in its immediate intravascular habitat as a survival mechanism.
Keywords: Anticoagulant; Dirofilaria immitis; coagulation cascade; excretory/secretory antigens; factor Xa; serpin.
Figures



References
-
- Adams RL and Bird RJ (2009) Review article: coagulation cascade and therapeutics update: relevance to nephrology. Part 1: overview of coagulation, thrombophilias and history of anticoagulants. Nephrology (Carlton) 14, 462–470. - PubMed
-
- Arneth B (2019) Coevolution of the coagulation and immune systems. Inflammation Research 68, 117–123. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G and Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology 340, 783–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

