Rab35-GEFs, DENND1A and folliculin differentially regulate podocalyxin trafficking in two- and three-dimensional epithelial cell cultures
- PMID: 31992598
- PMCID: PMC7076212
- DOI: 10.1074/jbc.RA119.011646
Rab35-GEFs, DENND1A and folliculin differentially regulate podocalyxin trafficking in two- and three-dimensional epithelial cell cultures
Abstract
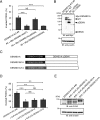
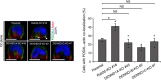
Polarized epithelial cells have functionally distinct apical and basolateral membranes through which they communicate with external and internal bodily environments, respectively. The establishment and maintenance of this asymmetric structure depend on polarized trafficking of specific cargos, but the precise molecular mechanism is incompletely understood. We previously showed that Rab35, a member of the Rab family small GTPases, differentially regulates the trafficking of an apical cargo, podocalyxin (PODXL), in two-dimensional (2D) and three-dimensional (3D) Madin-Darby canine kidney (MDCK) II cell cultures through specific interactions with two distinct effectors, OCRL inositol polyphosphate-5-phosphatase (OCRL) and ArfGAP with coiled-coil, ankyrin repeat and pleckstrin homology domains 2 (ACAP2), respectively. However, whether the upstream regulators of Rab35 also differ depending on the culture conditions remains completely unknown. Here, we investigated four known guanine nucleotide exchange factors (GEFs) of Rab35, namely DENN domain-containing 1A (DENND1A), DENND1B, DENND1C, and folliculin (FLCN), and demonstrate that DENND1A and FLCN exhibit distinct requirements for Rab35-dependent PODXL trafficking under the two culture conditions. In 3D cell cultures, only DENDN1A-knockout cysts exhibited the inverted localization of PODXL similar to that of Rab35-knockout cysts. Moreover, the DENN domain, harboring GEF activity toward Rab35, was required for proper PODXL trafficking to the apical membrane. By contrast, FLCN-knockdown cells specifically accumulated PODXL in actin-rich structures similar to the Rab35-knockdown cells in 2D cell cultures. Our findings indicate that two distinct functional cascades of Rab35, the FLCN-Rab35-OCRL and the DENND1A-Rab35-ACAP2 axes, regulate PODXL trafficking in 2D and 3D MDCK II cell cultures, respectively.
Keywords: 3D cell culture; Rab; cell polarity; epithelial cell; guanine nucleotide exchange factor (GEF); membrane trafficking; podocalyxin; small GTPase; tissue development.
© 2020 Kinoshita et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

