CA19-9 for detecting recurrence of pancreatic cancer
- PMID: 31992753
- PMCID: PMC6987233
- DOI: 10.1038/s41598-020-57930-x
CA19-9 for detecting recurrence of pancreatic cancer
Abstract
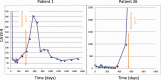
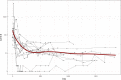
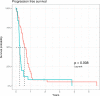
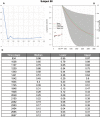
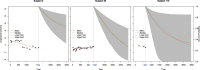
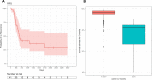
CA19-9 values are regularly measured in patients with pancreatic cancer. Certainly, its potential as a biomarker has been compromised by false negative results in CA19-9 negative patients and false positive results in benign pancreatico-biliary diseases. For detection of PDAC recurrence, however, CA19-9 might play an important role. The aim of this study is to analyze the accuracy of CA19-9 for detecting recurrence of pancreatic cancer. All included patients were treated either at the University Medical Center Goettingen, or at the Department of Interdisciplinary Oncology and Pneumonology, DRK-Kliniken Nordhessen, Kassel. We analyzed data of 93 patients with pancreatic cancer in the training set and 41 in the validation set, both retrospectively. Pre- and postoperative CA19-9 values and results of imaging techniques were compared. We performed ROC-analysis. The association between longitudinally measured CA19-9 values and relapse was studied with a joint model between a random effects model for the longitudinal CA19-9 measurements and a Cox proportional hazards models for the survival data. In the test set (n = 93 patients) the median follow-up time was 644 days (22 months). Overall, 71 patients (76.3%) developed recurrence during follow-up. Patients with CA19-9 values of <10kU/l were considered as CA19-9 negative patients (n = 11) and excluded from further analysis. Among the rest, approximately 60% of the patients showed significantly elevated CA19-9 prior to detection of recurrence by imaging techniques. Recurrence was shown by 2.45 times elevated CA19-9 values with 90% positive predictive value. In the validation set, 2.45 times elevated CA19-9 values showed recurrence with 90% sensitivity and 83,33% specificity, with an area under the curve of 95%. Based on measured CA19-9 values during follow-up care, the joint model estimates in recurrence-free patients the probability of recurrence-free survival. CA19-9 elevation is an early and reliable sign for PDAC recurrence. On the strength of a very high accuracy in CA19-9 positive patients, it should be considered to use CA19-9 for therapy decision even without a correlate of imaging technics. Using the joint model, follow-up care of PDAC patients after curative therapy can be stratified.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Magnani JL, et al. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J. Biol. Chem. 1982;257:14365–14369. - PubMed
-
- Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489–5492. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

