Synergistic anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and absolute stereochemistry of 7,8-dideoxygriseorhodin C
- PMID: 31992865
- PMCID: PMC7125055
- DOI: 10.1038/s41429-019-0275-8
Synergistic anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and absolute stereochemistry of 7,8-dideoxygriseorhodin C
Abstract
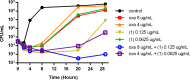
The emergence of antibiotic resistance necessitates not only the identification of new compounds with antimicrobial properties, but also new strategies and combination therapies to circumvent this growing problem. Here, we report synergistic activity against methicillin-resistant Staphylococcus aureus (MRSA) of the β-lactam antibiotic oxacillin combined with 7,8-dideoxygriseorhodin C in vitro. Ongoing efforts to identify antibiotics from marine mollusk-associated bacteria resulted in the isolation of 7,8-dideoxygriseorhodin C from a Streptomyces sp. strain cultivated from a marine gastropod tissue homogenate. Despite the long history of 7,8-dideoxygriseorhodin C in the literature, the absolute configuration has never been previously reported. A comparison of measured and calculated ECD spectra resolved the configuration of the spiroketal carbon C6, and 2D ROESY NMR spectroscopy established the absolute configuration as 6s,6aS. The compound is selective against Gram-positive bacteria including MRSA and Enterococcus faecium with an MIC range of 0.125-0.5 μg ml-1. Moreover, the compound synergizes with oxacillin against MRSA as observed in the antimicrobial microdilution and time-kill assays. Simultaneous treatment of the compound with oxacillin resulted in an approximately tenfold decrease in MIC with a combination index of <0.5, indicating synergistic anti-MRSA activity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

