Non-neutralizing Antibodies Directed at Conservative Influenza Antigens
- PMID: 31993232
- PMCID: PMC6977952
- DOI: 10.32607/20758251-2019-11-4-22-32
Non-neutralizing Antibodies Directed at Conservative Influenza Antigens
Abstract
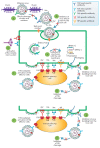
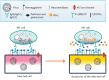
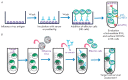
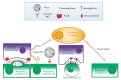
At the moment, developing new broad-spectrum influenza vaccines which would help avoid annual changes in a vaccine's strain set is urgency. In addition, developing new vaccines based on highly conserved influenza virus proteins could allow us to better prepare for potential pandemics and significantly reduce the damage they cause. Evaluation of the humoral response to vaccine administration is a key aspect of the characterization of the effectiveness of influenza vaccines. In the development of new broad-spectrum influenza vaccines, it is important to study the mechanisms of action of various antibodies, including non-neutralizing ones, as well as to be in the possession of methods for quantifying these antibodies after immunization with new vaccines against influenza. In this review, we focused on the mechanisms of anti-influenza action of non-neutralizing antibodies, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and antibody-mediated complement-dependent cytotoxicity (CDC). The influenza virus antigens that trigger these reactions are hemagglutinin (HA) and neuraminidase (NA), as well as highly conserved antigens, such as M2 (ion channel), M1 (matrix protein), and NP (nucleoprotein). In addition, the mechanisms of action and methods for detecting antibodies to neuraminidase (NA) and to the stem domain of hemagglutinin (HA) of the influenza virus are considered.
Keywords: antibody-dependent cellular cytotoxicity; antibody-dependent cellular phagocytosis; antibody-mediated complement-dependent cytotoxicity; broad-spectrum influenza vaccine; influenza virus.
Copyright ® 2019 National Research University Higher School of Economics.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous
