Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging
- PMID: 31993254
- PMCID: PMC6985772
- DOI: 10.1089/wound.2019.1021
Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging
Abstract
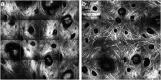
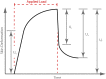
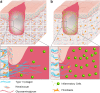
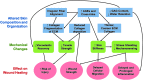
Significance: Chronic wounds, such as diabetic foot ulcers, venous stasis ulcers, and pressure ulcers affect millions of Americans each year, and disproportionately afflict our increasingly older population. Older individuals are predisposed to wound infection, repeated trauma, and the development of chronic wounds. However, a complete understanding of how the attributes of aging skin affect the wound healing process has remained elusive. Recent Advances: A variety of studies have demonstrated that the dermal matrix becomes thinner, increasingly crosslinked, and fragmented with advanced age. These structural changes, as well as an increase in cell senescence, result in altered collagen fiber remodeling and increased stiffness. Studies combining mechanical testing with advanced imaging techniques are providing new insights into the relationships between these age-related changes. Emerging research into the mechanobiology of aging and the wound healing process indicate that the altered mechanical environment of aged skin may have a significant effect on age-related delays in healing. Critical Issues: The interpretation and synthesis of clinical studies is confounded by the effects of common comorbidities that also contribute to the development of chronic wounds. A lack of quantitative biomarkers of wound healing and age-related changes makes understanding structure-function relationships during the wound healing process challenging. Future Directions: Additional work is needed to establish quantitative and mechanistic relationships among age-related changes in the skin microstructure, mechanical function, and the cellular responses to wound healing.
Keywords: collagen; mechanics; stiffness.
Copyright 2020, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Figures






References
-
- Harn HI, Ogawa R, Hsu CK, Hughes MW, Tang MJ, Chuong CM. The tension biology of wound healing. Exp Dermatol 2019;28:464–471 - PubMed
-
- Lavker RM, Zheng PS, Dong G. Morphology of Aged Skin. Dermatol Clin 1986;4:379–389 - PubMed
-
- Grahame R, Holt PJ. The influence of ageing on the in vivo elasticity of human skin. Gerontologia 1969;15:121–139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

