KRAS mutant tenosynovial giant cell tumor in a pediatric patient: a case report
- PMID: 31993359
- PMCID: PMC6970126
- DOI: 10.21037/tp.2019.11.05
KRAS mutant tenosynovial giant cell tumor in a pediatric patient: a case report
Abstract
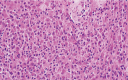
Tenosynovial giant cell tumors (TSGCT) are a group of rare, benign soft tissue tumors with common histologic and cytogenetic features, with a median age of diagnosis being 47 years. Generally divided into localized and diffuse subtypes, TSGCTs are typically driven by overexpression of macrophage colony stimulating factor receptor-1 (CSF1R). Treatment of TSGCT is tumor resection, followed by radiation therapy in cases of incomplete resection. Even when the tumor is completely removed, recurrence rates can be as high as 30% in some anatomical locations. Here we report the identification of a previously undescribed KRAS p.G12D activating mutation within a pediatric TSGCT patient, who clinically presented with an enlarging right lower extremity mass pathologically consistent with TSGCT. The patient continues to be in remission three years after complete surgical removal. KRAS mutations are usually found in adult cancers, such as lung and pancreatic, as well as giant cell lesion of the jaw. This case demonstrates the utility of integrative clinical sequencing in identifying lesions with aggressive potential and aiding in complex diagnoses.
Keywords: KRAS; case report; integrative clinical sequencing; pediatric oncology; tenosynovial giant cell tumor (TSGCT).
2019 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: R.Mody is a Hyundai Hope on Wheels Scholar. The other authors have no conflicts of interest to declare.
Figures






Similar articles
-
Diagnostic utility of CSF1 immunohistochemistry in tenosynovial giant cell tumor for differentiating from giant cell-rich tumors and tumor-like lesions of bone and soft tissue.Diagn Pathol. 2022 Nov 1;17(1):88. doi: 10.1186/s13000-022-01266-9. Diagn Pathol. 2022. PMID: 36320082 Free PMC article.
-
Optimal Treatment for Tenosynovial Giant Cell Tumor of the Hand.J Nippon Med Sch. 2020 Sep 9;87(4):184-190. doi: 10.1272/jnms.JNMS.2020_87-408. Epub 2020 Apr 30. J Nippon Med Sch. 2020. PMID: 32350187 Review.
-
Clinical and morphological correlations and histopathology of joint damage in patients with diffuse-type tenosynovial giant cell tumor.Wiad Lek. 2019;72(12 cz 1):2269-2276. Wiad Lek. 2019. PMID: 32124739
-
Massively parallel sequencing of tenosynovial giant cell tumors reveals novel CSF1 fusion transcripts and novel somatic CBL mutations.Int J Cancer. 2019 Dec 15;145(12):3276-3284. doi: 10.1002/ijc.32421. Epub 2019 May 31. Int J Cancer. 2019. PMID: 31107544
-
Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI?Cancers (Basel). 2024 Jan 17;16(2):402. doi: 10.3390/cancers16020402. Cancers (Basel). 2024. PMID: 38254890 Free PMC article. Review.
References
-
- de Saint Aubain Somerhausen N, van de Rijn M. Tenosynovial giant cell tumour, localized type Tenosynovial giant cell tumour, diffuse type. In: Fletcher CD, Bridge JA, Hogendoorn P, et al. (eds) World Health Organization classification of tumours of soft tissue and bone. Lyon: IARC, 2013:100-3.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
